1. Gregory JM, Slaughter JC, Duffus SH, Smith TJ, LeStourgeon LM, Jaser SS, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021; 44(2):526–532. PMID:
33268335.
2. COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Coronavirus disease-19: the first 7,755 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020; 11(2):85–90. PMID:
32257774.
3. Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-onset diabetes in COVID-19. N Engl J Med. 2020; 383(8):789–790. PMID:
32530585.
4. Chung SM, Lee YY, Ha E, Yoon JS, Won KC, Lee HW, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. 2020; 44(3):405–413. PMID:
32602272.
5. Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes Metab J. 2020; 44(2):349–353. PMID:
32347027.
6. Ku CR, Jung KY, Ahn CH, Moon JS, Lee JH, Kim EH, et al. COVID-19 vaccination for endocrine patients: a position statement from the Korean Endocrine Society. Endocrinol Metab. 2021; 36(4):757–765.
7. Kim IC, Kim H, Lee HJ, Kim JY, Kim JY. Cardiac imaging of acute myocarditis following COVID-19 mRNA vaccination. J Korean Med Sci. 2021; 36(32):e229. PMID:
34402228.
8. Lim KH, Park JS. COVID-19 vaccination-induced ventricular fibrillation in an afebrile patient with Brugada syndrome. J Korean Med Sci. 2022; 37(42):e306. PMID:
36325610.
9. Finsterer J, Matovu D. Consider transverse myelitis as a complication of a SARS-CoV-2 vaccination. J Korean Med Sci. 2022; 37(18):e150. PMID:
35535377.
10. Edwards AE, Vathenen R, Henson SM, Finer S, Gunganah K. Acute hyperglycaemic crisis after vaccination against COVID-19: a case series. Diabet Med. 2021; 38(11):e14631. PMID:
34185927.
11. Lee HJ, Sajan A, Tomer Y. Hyperglycemic emergencies associated with COVID-19 vaccination: a case series and discussion. J Endocr Soc. 2021; 5(11):bvab141. PMID:
34604689.
12. Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med. 1989; 320(14):881–886. PMID:
2648146.
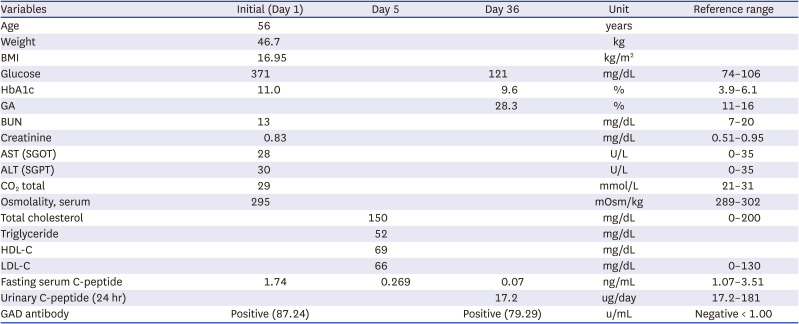
13. VanBuecken DE, Greenbaum CJ. Residual C-peptide in type 1 diabetes: what do we really know? Pediatr Diabetes. 2014; 15(2):84–90. PMID:
24645775.
14. Cuschieri S, Grech S. COVID-19 and diabetes: the why, the what and the how. J Diabetes Complications. 2020; 34(9):107637. PMID:
32456846.
15. Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020; 14(6):2211–2217. PMID:
33395782.
16. Sakurai K, Narita D, Saito N, Ueno T, Sato R, Niitsuma S, et al. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J Diabetes Investig. 2022; 13(7):1290–1292.
17. Yano M, Morioka T, Natsuki Y, Sasaki K, Kakutani Y, Ochi A, et al. New-onset type 1 diabetes after COVID-19 mRNA vaccination. Intern Med. 2022; 61(8):1197–1200. PMID:
35135929.
18. Watad A, Bragazzi NL, McGonagle D, Adawi M, Bridgewood C, Damiani G, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: insights from an analysis of 500 cases. Clin Immunol. 2019; 203:1–8. PMID:
30922961.
19. Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021; 3(2):149–165. PMID:
33536639.
20. Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2021; 47(2):101204. PMID:
33129968.
21. Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009; 302(2):193–202. PMID:
18948167.
22. Blum SI, Tse HM. Innate viral sensor MDA5 and coxsackievirus interplay in type 1 diabetes development. Microorganisms. 2020; 8(7):993. PMID:
32635205.