Abstract
Metallic implant-associated lymphomas are extremely rare. Only seven cases have been reported in association with knee joint arthroplasty, and all tumors were large B-cell lymphomas. This report is the first case of anaplastic large cell lymphoma occurring after total knee replacement arthroplasty. An 80-year-old female patient was admitted because of right knee pain for 2 years. She had undergone total knee replacement arthroplasty 10 years prior. Computed tomography showed an irregular osteolytic lesion in the right lateral femoral condyle, adjacent to the metallic prosthesis. Histologic findings reveal sheets of anaplastic tumor cells that were positive for CD2, CD4, CD5, CD43, and CD30 but negative for CD3, CD20, CD15, and anaplastic lymphoma kinase. Epstein-Barr encoding region in situ hybridization was negative. Analysis of T-cell receptor γ gene rearrangement studies using BIOMED-2–based multiplex polymerase chain reaction confirmed monoclonal T cell proliferation. The woman was finally diagnosed with ALK-negative anaplastic large cell lymphoma.
Primary lymphoma of the bone and joint is rare. Malignant lymphomas related to metallic implants from an orthopedic procedure, called metallic implant-associated lymphomas, are extremely rare [1]. Only seven cases of malignant lymphomas complicating total knee replacement arthroplasty have been reported and occur several years after operation; all cases were large B-cell lymphoma [1–7].
Herein, we describe the first reported case of anaplastic large cell lymphoma (ALCL) presenting as periprosthetic joint infection occurring 10 years after total knee replacement arthroplasty, suggesting that patients with an inflammatory response to orthopedic prostheses should be monitored carefully for an extended time.
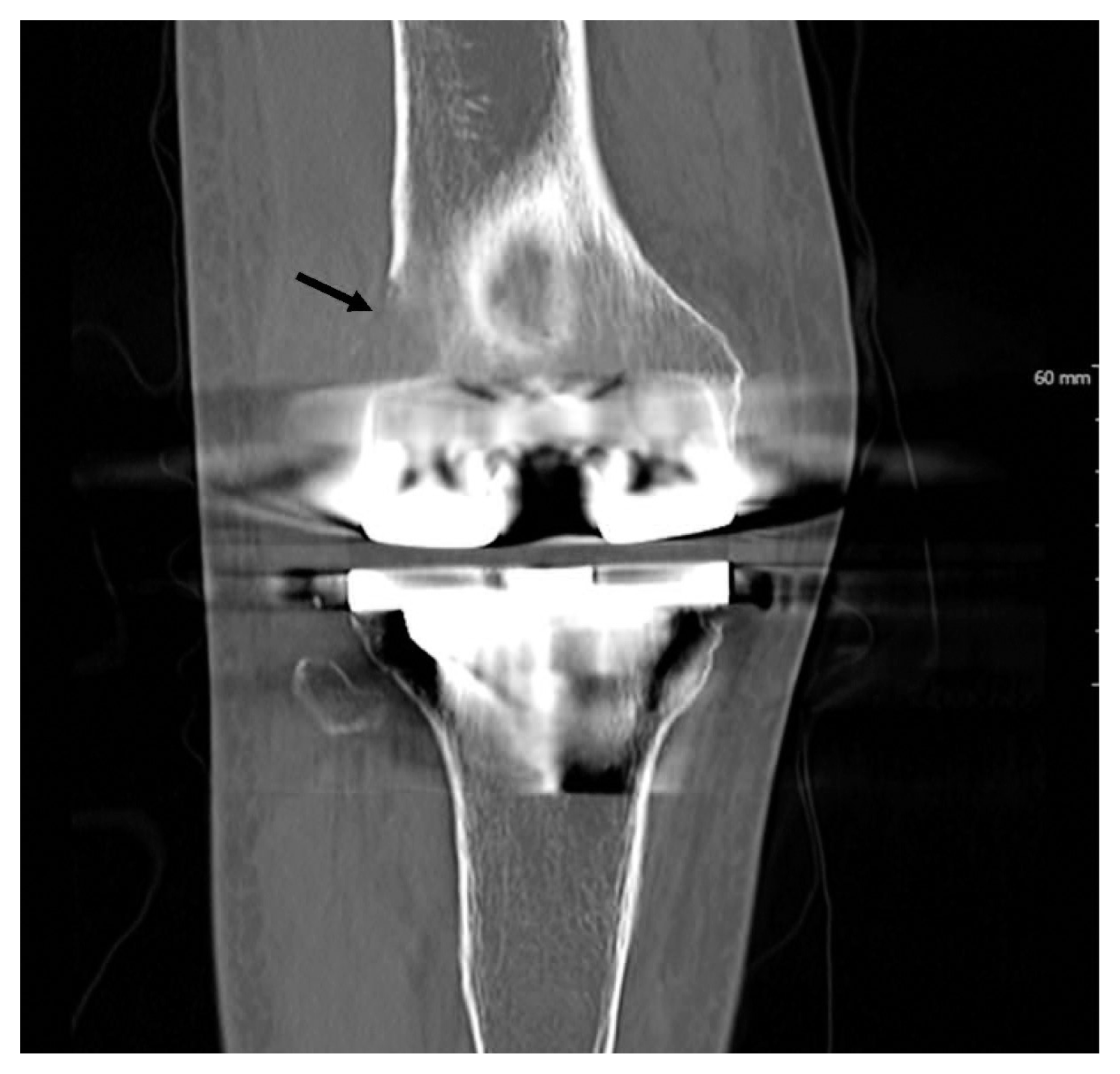
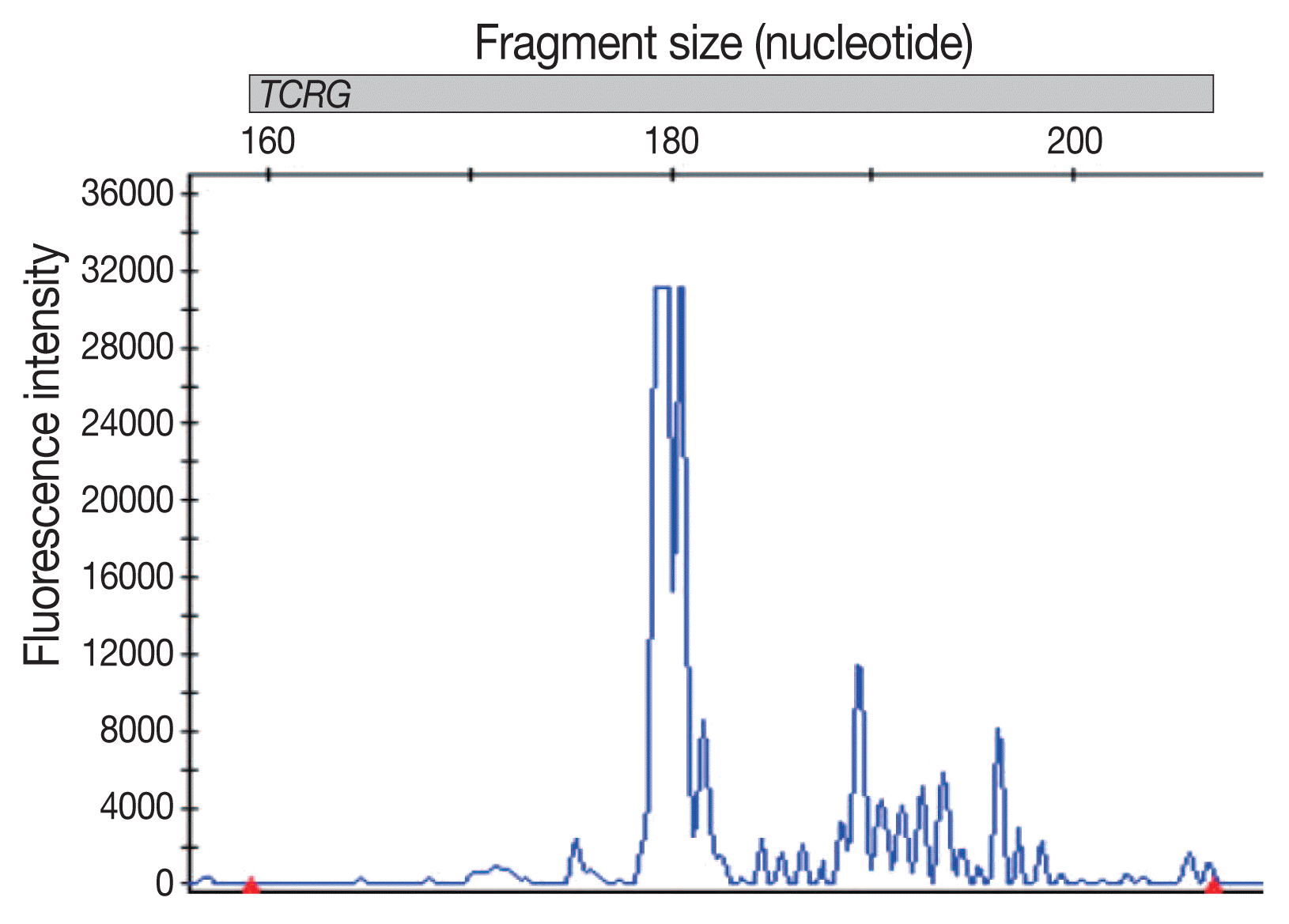
An 80-year-old female patient was admitted because of right knee pain for 2 years. The pain had recently increased, although there was no history of trauma. She had undergone total knee replacement arthroplasty 10 years prior. Computed tomography demonstrated an irregular osteolytic mass-like lesion in the right lateral femoral condyle, adjacent to the metallic prosthesis, and a large amount of joint effusion with periarticular soft tissue swelling (Fig. 1), which suggested metallosis, or aseptic lymphocyte-dominant vasculitis-associated lesion, and infectious arthritis. Arthrotomy, hardware removal, and anti-cement insertion were performed under the clinical impression of septic knee. Intraoperatively, synovial hypertrophy, inflammatory change, necrotic tissue, and a mass-like lesion were observed in the right knee joint. Histologically, several fragments of bone and soft tissues were composed of sheets of anaplastic tumor cells, which had irregularly folded nuclei, prominent nucleoli, and a moderate amount of amphophilic cytoplasm (Fig. 2A). Most of the tumor cells were positive for CD30 (Fig. 2B). The tumor cells were also positive for CD2, CD4 (Fig. 2C), CD5, CD43 (Fig. 2D), epithelial membrane antigen, TIA-1, perforin, and granzyme B but negative for CD3, CD7, CD8, CD20, Pax5, CD15, CD68, lysozyme, CD1a, and S100. These cells did not express anaplastic lymphoma kinase (ALK). Epstein-Barr encoding region in situ hybridization was negative. Analysis of T-cell receptor γ gene rearrangement studies using BIOMED-2–based multiplex polymerase chain reaction confirmed monoclonal T-cell proliferation (Fig. 3). The woman was finally diagnosed with ALK-negative ALCL. Whole body bone scan showed high 18F-fluorodeoxyglucose uptake in bone and synovium at the right knee arthroplasty removal site, with overlying soft tissue inflammation and joint effusion. There was no evidence of other site involvement in systemic workup, consistent with stage 1 disease. Postoperative definite radiotherapy was administered, and the patient was in good health at the latest follow-up (1 year). Follow-up position emission tomography–computed tomography (PET-CT) revealed no residual mass.
Medical devices such as breast, hip, knee, and vascular prostheses can be associated with malignant lymphomas. A prototype is an entity related to breast implants, called breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), and more than 300 cases have been described in the literature [8]. Genomic characterization of BIA-ALCL shows abnormalities similar to those of systemic ALCL [8]. The characteristic clinical features are late-onset implant-associated seroma occurring greater than 1-year post-operative and an indolent course. Most patients are cured by implant removal [9]. In addition to ALCL, other types of lymphomas have been reported to be associated with breast implants [9]. To date, 28 cases of implant-associated B-cell lymphoma have been reported, 18 of which were diffuse large B-cell lymphoma (DLBCL) [10]. Although the available data regarding causation and treatment are limited in BIA-DLBCL, the treatments of BIA-ALCL and DLBCL might be similar. The majority of these two different lymphoma types is well-localized and show similar favorable clinical courses [10].
A prosthesis may act as an immune adjuvant [9], and an immune reaction to the prosthetic material may cause T cell infiltration with later clonal expansion of T lymphocytes. A reaction to biofilms can generate a response by stimulating toll-like receptors on immune cells. Bacterial cell-wall components are potent stimulators of these responses. Contamination of the implant with bacterial fragments can maintain a chronic inflammatory response [8,9]. In contrast, the implants elicit chronic immune system stimulation against the prosthetic material, particularly in genetically susceptible hosts. Therefore, polyclonal activation leads to autoantibody formation, polyclonal hypergammaglobulinemia progressing to monoclonality, and B-cell lymphoma in at-risk hosts [9].
Metallic implant-associated lymphomas are exceedingly rare [1], with only a few cases reported. This raises suspicion of possible oncogenic properties of such materials [9]. The wear debris of implanted synthetic materials is not biologically inert [11], and microparticle detachment from implants might be involved in lymphoproliferative responses and is an important factor in the development of lymphomas [8]. Metallic implant-associated lymphoma is thought to resemble lymphomas associated with other chronic inflammatory conditions such as chronic osteomyelitis and pyothorax, and the two share several clinicopathologic features including development in the setting of prolonged chronic inflammation, localization to a confined body space, a long latency period, and the presence of large cell phenotype [11]. Most metallic implant-associated lymphomas were of B-cell origin, in contrast to breast implants. To the best of my knowledge, only one case of ALCL for a tibial metal implant has been published in the English literature [11], and another case of metallic dental implant-associated mucosal CD30-positive T-cell lymphoproliferative disorder was reported in Korea [12].
Only seven cases of malignant lymphomas complicating total knee replacement arthroplasty have been reported in the medical literature [1–7]. The mean time between implantation of the prostheses and lymphoma diagnosis was 7 years (range, 6 months to 16 years). All tumors were classified as large B-cell lymphomas. All cases arose in the bone and five of them were found in wear debris adjacent to the prosthesis or periprosthetic membrane. Three cases were presented as osteolytic bone lesions with soft tissue extension as is the case described above. Four patients were treated with radiotherapy and chemotherapy, one was treated with radiotherapy, one was treated with chemotherapy, and one patient was not treated. Follow-up was reported in six patients, all of them being free of disease for 8 months to 3 years. In particular, one patient was alive for 2 years without evidence of disease, even though no additional treatment was received other than revision arthroplasty [6].
In conclusion, this is the first reported case of ALCL complicating total knee replacement arthroplasty, which can cause prosthesis failure long after the placement operation. Periprosthetic primary lymphoma of the bone should be included in the differential diagnosis of a patient presenting with knee pain, knee mass, or lytic destruction after knee arthroplasty [5].
Notes
Ethics Statement
The Institutional Review Board of Dankook University Hospital (2022-03-028) approved this case report and informed consent was waived.
References
1. Cheuk W, Chan AC, Chan JK, Lau GT, Chan VN, Yiu HH. Metallic implant-associated lymphoma: a distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005; 29:832–6.
2. Chaudhry MS, Mather H, Marks A, Naresh K. Diffuse large B cell lymphoma complicating total knee arthroplasty: case report and literature review of the association of diffuse large B cell lymphoma with joint replacement. Acta Haematol. 2011; 126:141–6.

3. Sanchez-Gonzalez B, Garcia M, Montserrat F, et al. Diffuse large B-cell lymphoma associated with chronic inflammation in metallic implant. J Clin Oncol. 2013; 31:e148–51.

4. Sunitsch S, Gilg M, Kashofer K, Leithner A, Liegl-Atzwanger B, Beham-Schmid C. Case report: Epstein-Barr-virus negative diffuse large B-cell lymphoma detected in a peri-prosthetic membrane. Diagn Pathol. 2016; 11:80.

5. Ibrahim I, Haughom BD, Fillingham YA, Brown N, Gitelis S. Primary lymphoma of bone complicating total knee arthroplasty: an unexpected mode of prosthesis failure: a case report. JBJS Case Connect. 2015; 5:e34.
6. Hall J, Kampfer C, Williams N, et al. Fibrin-associated diffuse large B cell lymphoma found on revision arthroplasty of the knee. South Med J. 2021; 114:708–13.

7. Agrawal K, Agrawal N, Levin M. Primary synovial diffuse large B-cell lymphoma presenting as loosening of prosthetic joint: a case report and review of literature. World J Oncol. 2019; 10:181–5.

8. Ramos-Gallardo G, Carballo-Zarate AA, Cuenca-Pardo J, et al. What is the evidence of lymphoma in patients with prostheses other than breast implants? Aesthetic Plast Surg. 2020; 44:286–94.

9. Bizjak M, Selmi C, Praprotnik S, et al. Silicone implants and lymphoma: the role of inflammation. J Autoimmun. 2015; 65:64–73.

10. Morgan S, Tremblay-LeMay R, Lipa JE, et al. Breast implant-associated EBV-positive diffuse large B-cell lymphoma: two case reports and literature review. Pathol Res Pract. 2021; 226:153589.

Fig. 1
Computed tomography shows an irregular osteolytic mass-like lesion (arrow) in the right lateral femoral condyle, adjacent to the metallic prosthesis.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download