1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization website. Updated 2022. Accessed October 23, 2022.
https://covid19.who.int/info
.
2. Adjei P, Afriyie-Mensah J, Ganu VJ, Puplampu P, Opoku-Asare B, Dzefi-Tettey K, et al. Clinical characteristics of COVID-19 patients admitted at the Korle-Bu Teaching Hospital, Accra, Ghana. Ghana Med J. 2020; 54(4):Suppl. 33–38. PMID:
33976439.
3. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID:
32242348.
4. Kim M, Yoo JR, Heo ST, Lee HR, Oh H. Clinical characteristics and risk factors for severe disease of coronavirus disease 2019 in a low case fatality rate region in Korea. Infect Chemother. 2021; 53(4):718–729. PMID:
34951535.
6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13):1239–1242. PMID:
32091533.
7. Shin HS. Empirical treatment and prevention of COVID-19. Infect Chemother. 2020; 52(2):142–153. PMID:
32476308.
9. Kim SB, Kim J, Huh K, Choi WS, Kim YJ, Joo EJ, et al. Korean Society of Infectious Diseases/national evidence-based healthcare collaborating agency recommendations for anti-SARS-CoV-2 monoclonal antibody treatment of patients with COVID-19. Infect Chemother. 2021; 53(2):395–403. PMID:
34216134.
10. Kim SB, Ryoo S, Huh K, Joo EJ, Kim YJ, Choi WS, et al. Revised Korean Society of Infectious Diseases/national evidence-based healthcare collaborating agency guidelines on the treatment of patients with COVID-19. Infect Chemother. 2021; 53(1):166–219. PMID:
34409790.
12. Kim SB, Huh K, Heo JY, Joo EJ, Kim YJ, Choi WS, et al. Interim guidelines on antiviral therapy for COVID-19. Infect Chemother. 2020; 52(2):281–304. PMID:
32342676.
13. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004; 323(1):264–268. PMID:
15351731.
14. Hong KS, Jang JG, Hur J, Lee JH, Kim HN, Lee W, et al. Early hydroxychloroquine administration for rapid severe acute respiratory syndrome coronavirus 2 eradication. Infect Chemother. 2020; 52(3):396–402. PMID:
32757497.
15. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020; 383(21):2030–2040. PMID:
33031652.
16. Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial. Clin Infect Dis. 2021; 73(11):e4073–e4081. PMID:
32674126.
17. Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson J, et al. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020; 396(10259):1345–1352. PMID:
33031764.
18. Hoang T, Anh TT. Treatment options for severe acute respiratory syndrome, middle east respiratory syndrome, and coronavirus disease 2019: a review of clinical evidence. Infect Chemother. 2020; 52(3):317–334. PMID:
32869558.
19. Lee C, Ahn MY, Byeon K, Choi JP, Hahm C, Kim H, et al. Clinical experience with use of remdesivir in the treatment of severe acute respiratory syndrome coronavirus 2: a case series. Infect Chemother. 2020; 52(3):369–380. PMID:
32757500.
20. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-final report. N Engl J Med. 2020; 383(19):1813–1826. PMID:
32445440.
21. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395(10236):1569–1578. PMID:
32423584.
22. Joo EJ, Ko JH, Kim SE, Kang SJ, Baek JH, Heo EY, et al. Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study. J Korean Med Sci. 2021; 36(11):e83. PMID:
33754512.
23. Choi JY. Convalescent plasma therapy for coronavirus disease 2019. Infect Chemother. 2020; 52(3):307–316. PMID:
32989938.
24. Baek AR, Choo EJ, Kim JY, Ha TS, Park SW, Shin HB, et al. A transient effect of convalescent plasma therapy in a patient with severe covonavirus disease 2019: a case report. Infect Chemother. 2022; 54(3):553–558. PMID:
35920265.
25. Im JH, Nahm CH, Baek JH, Kwon HY, Lee JS. Convalescent plasma therapy in coronavirus disease 2019: a case report and suggestions to overcome obstacles. J Korean Med Sci. 2020; 35(26):e239. PMID:
32627442.
26. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021; 384(8):693–704. PMID:
32678530.
27. Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021; 181(1):32–40. PMID:
33080017.
28. Mariette X, Hermine O, Tharaux PL, Resche-Rigon M, Steg PG, Porcher R, et al. Effectiveness of tocilizumab in patients hospitalized with COVID-19: a follow-up of the CORIMUNO-TOCI-1 randomized clinical trial. JAMA Intern Med. 2021; 181(9):1241–1243. PMID:
34028504.
29. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021; 384(9):795–807. PMID:
33306283.
30. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020; 18(5):1023–1026. PMID:
32338827.
31. Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021; 9(12):1365–1376. PMID:
34672949.
32. Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006; 354(16):1671–1684. PMID:
16625008.
33. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020; 8(3):267–276. PMID:
32043986.
34. Hong JY, Ko JH, Yang J, Ha S, Nham E, Huh K, et al. Severity-adjusted dexamethasone dosing and tocilizumab combination dosing and tocilizumab combination for severe COVID-19. Yonsei Med J. 2022; 63(5):430–439. PMID:
35512745.
35. Ejaz A, Ahmed MM, Tasleem A, Rafay Khan Niazi M, Ahsraf MF, Ahmad I, et al. Thromboprophylaxis in intensive care unit patients: a literature review. Cureus. 2018; 10(9):e3341. PMID:
30473974.
36. Peck KR. Collaborative response to COVID-19 pandemic, and development of treatment guidelines. Infect Chemother. 2021; 53(1):151–154. PMID:
34409788.
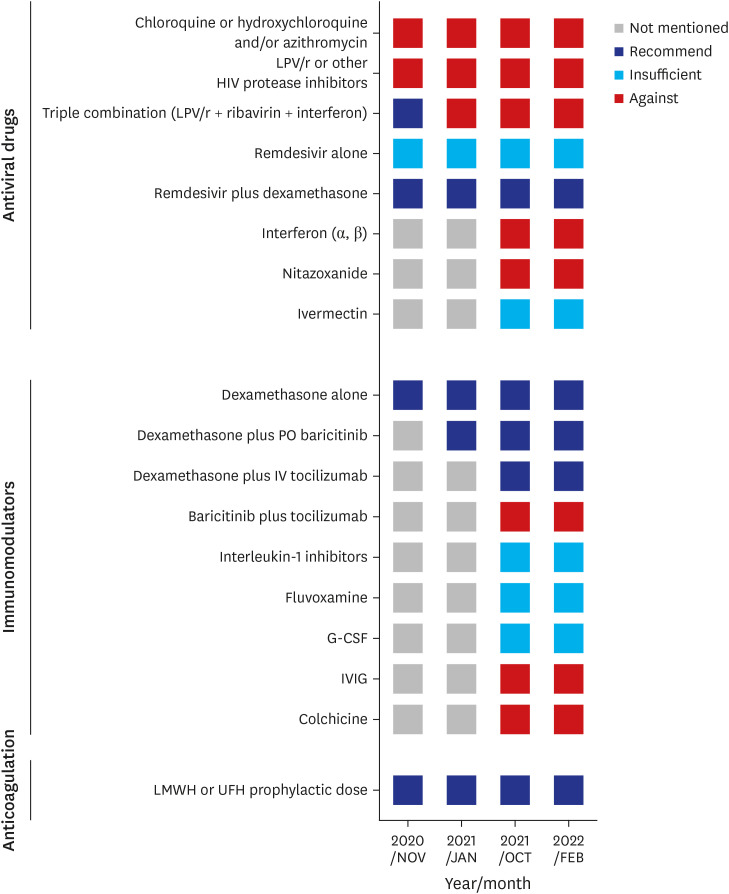
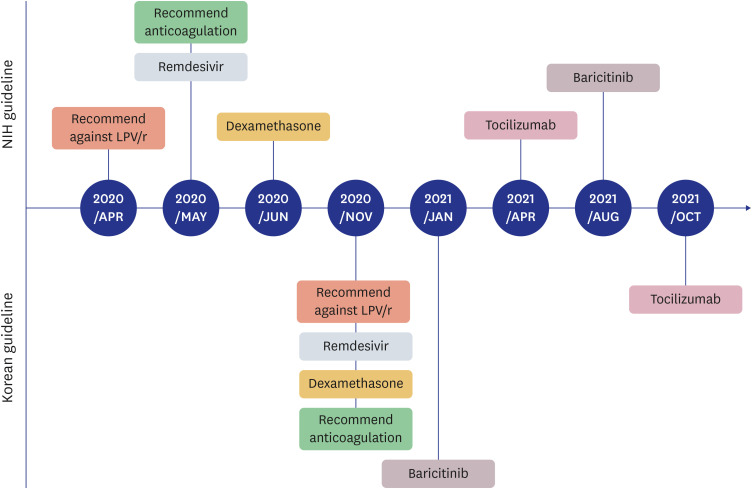
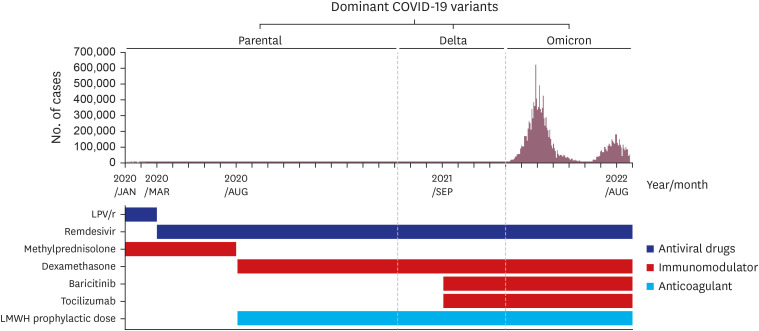




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download