INTRODUCTION
Atrial fibrillation (AF) is associated with an increased risk of thromboembolic events. Non-vitamin K antagonist oral anticoagulants (NOACs) are effective in preventing thromboembolisms and generally recommended as first-line therapy in preference to warfarin.
12 Furthermore, NOACs reduced the risk of major bleeding compared with warfarin in pivotal clinical trials.
3456 All NOACs are administered in fixed doses without need for coagulation monitoring and have individual dose reduction criteria. Dose reduction criteria were met by 5–25% of patients enrolled in a pivotal trial. In the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial, edoxaban was non-inferior to warfarin for stroke prevention and was superior to warfarin in safety outcome. In higher-dose regimens, the outcomes of a once-daily dose of edoxaban 30 mg in patients who met one of the criteria for dose reduction was investigated. Pharmacokinetic analysis in the ENGAGE AF-TIMI 48 trial showed consistent efficacy and safety of a reduced dose.
7 However, a meta-analysis investigating the outcomes of on-label reduced-dose NOACs showed that patients eligible for reduced dose were at a higher risk of both thromboembolism and bleeding complications and were characterized by low body weight, advanced age, and renal impairment.
8 These factors increased the risk of major bleeding and other adverse events.
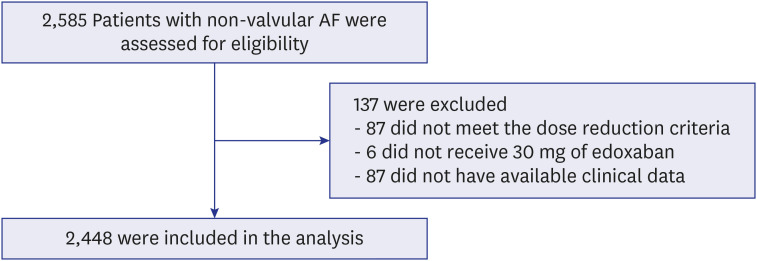
Nevertheless, there are few reports on the outcomes of on-label reduced-dose NOACs. In this study, we aimed to assess safety and efficacy of on-label reduced-dose edoxaban in East Asian patients with AF in real world practice.
DISCUSSION
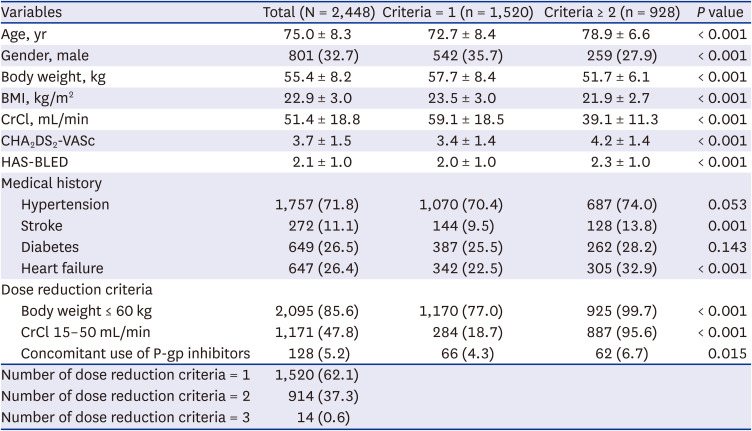
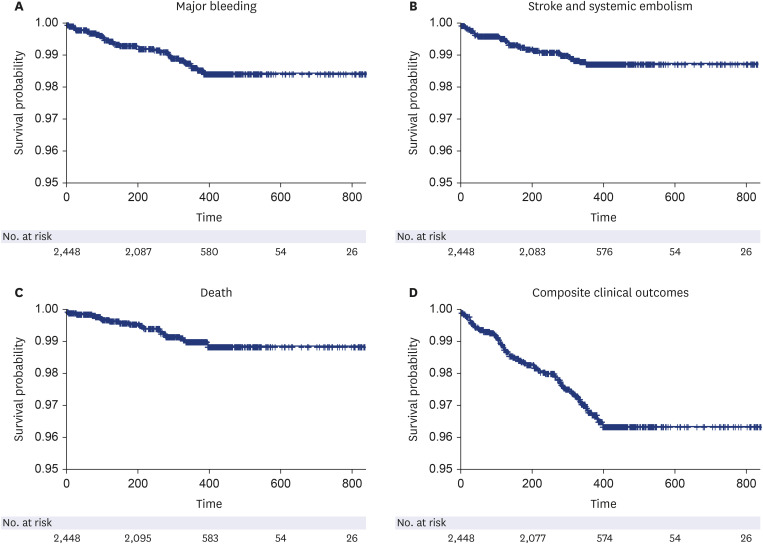
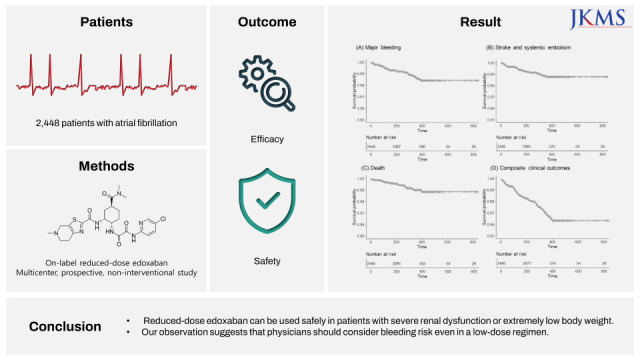
This study was the largest, prospective real-world study to investigate the safety and efficacy of on-label low-dose edoxaban in Asian patients. The main findings are as follows. First, on-label reduced dose of edoxaban is safe and effective in real-world practice. The clinical event rates for major bleeding, stroke, and net clinical outcome were low. Second, the results were consistent according to the dose reduction criteria or other baseline characteristics, including severe renal dysfunction or extremely low body weight. Third, higher bleeding score or combination of stroke and bleeding score showed unfavorable composite clinical outcomes.
The ENGAGE AF-TIMI 48 trial was a three-group, randomized trial comparing two dose regimens of edoxaban with warfarin, and a higher dose regimen was approved according to the result.
4 In a pivotal randomized controlled trial, 1,784 (25.4%) patients were eligible for reduced-dose edoxaban, and the annualized event rate was 1.79% for stroke or systemic embolic event (SEE) and 3.05% major bleeding. Higher rates of thromboembolic events and major bleeding outcomes were observed compared to those in the no dose reduction group.
7 Both groups showed better outcomes compared with the warfarin group. These results were consistent in meta-analysis of three pivotal randomized controlled trials (RCTs) comparing reduced dose of NOAC with warfarin in patients with AF.
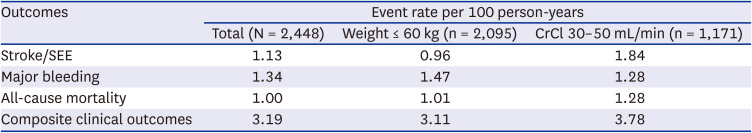
8 The annualized rate of major bleeding was 3.60% and the annualized rate of stroke or systemic embolism was 2.39% for patients eligible for reduced-dose NOACs, who had higher risk of thromboembolic and bleeding complications than those who did not experience dose reduction. In our study cohort, the overall major bleeding event rate was 1.34%, and that of stroke and SEE was 1.13%. In general, real-world studies have fewer events than randomized trials; even considering this, our study results demonstrated the safety and efficacy of on-label reduced-dose edoxaban in the real world.
In the ENGAGE AF-TIMI 48 trial, 684 patients received 30 mg of edoxaban due to body weight ≤ 60 kg, and 1,306 received reduced dose due to CrCl 30–50 mL/min in the higher-dose edoxaban group.
7 Outcomes stratified by dose reduction criteria in that pivotal trial were as follows. The event rate for major bleeding was 3.16%/yr and that for stroke was 2.05%/yr in patients with body weight ≤ 60 kg, and the event rate for major bleeding was 3.26%/yr and that for stroke was 1.74%/yr in patients with renal dysfunction. In our study cohort, we included a larger number of patients, and events rates were 1.47%/yr and 0.96%/yr for major bleeding and stroke, respectively, in low-body weight group, which showed favorable outcomes. In the renal dysfunction group, event rates were 1.28%/yr and 1.84%/yr for major bleeding and stroke, respectively. These results demonstrate the safety of reduced-dose edoxaban and showed comparable efficacy.
According to race, there were 2,909 patients of Asian and 18,195 of non-Asian in the pivotal trial, and Asian race was associated with favorable safety and comparable efficacy to warfarin.
10 A similar trend was also demonstrated in subgroup analysis of East Asians.
11 This may be due to the lower trough concentration and anti-FXa activity of the Asian race, and the increased risks of major bleeding on warfarin, especially intracranial hemorrhage, in the Asian population.
12 Only 294 East Asian individuals were included in the pivotal trial, and event rate for major bleeding was 1.91%/yr and that of stroke was 1.41%/yr.
11 Although our study is non-interventional, our results show comparable outcomes in both safety and efficacy, which suggests that reduced-dose edoxaban can be used safely and effectively in the Asian population. Further, the mean body weight and CrCl in pivotal trial was 65 kg and 46 mL/min. In our cohort, the mean body weight and CrCl was 55 kg and 51 mL/min. Our result demonstrated the efficacy and safety in low body weight population. We demonstrated this through large-scale, well-controlled real-world evidence.
Both efficacy and safety are important in oral anticoagulant therapy. The concern of fatal bleeding has resulted in overuse of off-label low-dose NOAC, especially in frail patients. However, off-label underdose of NOACs is associated with a higher risk of stroke in real-world evidence.
1314 Recently, there has been a report that compared the effects of lower dose edoxaban regimen (LDER) and higher dose edoxaban regimen (HDER) on the composite net clinical outcomes of stroke, systemic embolism, major bleeding, or all-cause mortality.
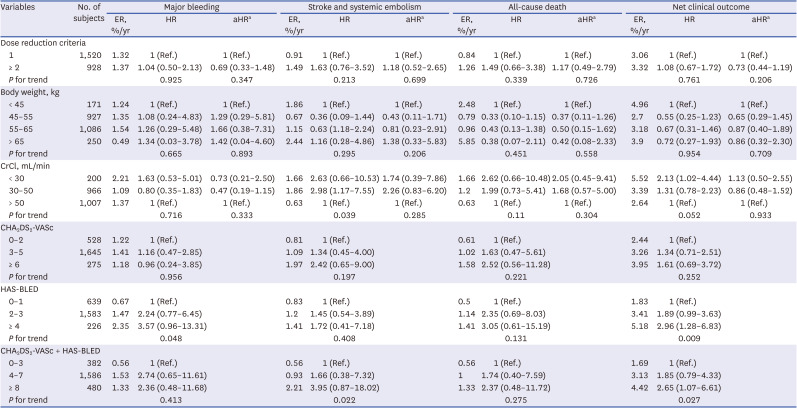
15 The primary net clinical outcome was reduced with LDER compared with HDER. The higher stroke events in the LDER group were counterbalanced by significantly fewer major bleeding events. The study concluded that LDER of edoxaban might be considered in high-bleeding risk patients. Our study cohort consisted of patients who were indicated for on-label reduced-dose edoxaban (HDER). Severe renal dysfunction or extremely low body weight showed no significant increased risks of all of the outcomes after adjusting for an age factor. Interestingly, a higher HAS-BLED score was associated with increased composite clinical outcomes. Higher combination of CHA
2DS
2-VASc and HAS-BLED score showed a similar result. The predictive power of both scores combined was significantly enhanced for mortality outcome compared to that of each score alone.
16 Therefore, our results support the conclusion of the previous study and indicate the importance of bleeding risk, even in an on-label reduced-dose protocol. Lee et al.
17 reported the benefits for edoxaban in real world Asian population. In subgroup analysis, edoxaban 30 mg (n = 2,371) showed similar net clinical benefit compared to warfarin. Our study demonstrated prospectively the benefits of on-label reduced dose edoxaban.
This study had some limitations. This study was a non-interventional single-arm observational study. Therefore, there was no control group, and it was difficult to directly compare the outcomes to those of a randomized control study. Nevertheless, our results indicated the safety and efficacy of on-label reduced-dose edoxaban in the real world. Second, this study included only a Korean population, representing a single ethnic group of East Asian. These results might not represent all races, who may have different characteristics from those of a non-Asian population. However, Asians have a large distribution of low body weight, and there are many Asian patients who are indicated for reduced-dose regimen. Therefore, our study result can be thought of as being applicable to a significant number of patients who need dose reduction. Finally, we were not able to measure the plasma concentrations or anti-factor Xa activity and could not determine a correlation of plasma concentration, anti-factor Xa activity, and occurrence of clinical outcomes.
In conclusion, this study revealed large-scale data on the safety and effectiveness of on-label reduced-dose edoxaban in an Asian population. On-label reduced-dose edoxaban can be used safely even in patients with severe renal dysfunction or extremely low body weight. Our observation suggests that physicians should consider bleeding risks, even in a low-dose regimen.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download