INTRODUCTION
Tuberculosis (TB) is a contagious disease caused by
Mycobacterium tuberculosis, which particularly affects the lungs and is easily spread through the air.
12 In response to active TB eradication programs, the global incidence rate and mortality rate for TB have been decreasing by 2% and 3%, respectively, every year.
34 However, TB remains one of the top 10 causes of death, taking the lead for a single infectious disease (pre-coronavirus disease era).
5 Moreover, as Korea has ranked with the highest TB incidence rate among the Organization for Economic Co-operation and Development countries, the Korean government established the first (2013–2017) and second (2018–2022) National Strategic Plans for TB with the goal of lowering the incidence rate to 40 per 100,000 by 2022.
678
Multidrug-resistant (MDR)-TB is resistant to at least isoniazid (INH) and rifampin (RMP), the backbone compounds of standard first-line TB treatments. Moreover, extensively drug-resistant-TB is more resistant to drugs than MDR-TB is, including INH, RMP, fluoroquinolones, and at least three injectable second-line drugs [amikacin (AMK), kanamycin, or capreomycin].
12 Because drug-resistant TB is resistant to the most potent TB drugs, the remaining treatment options are less effective, have more side effects, have longer treatment periods, and are more expensive; hence, their treatment success rate is extremely low,
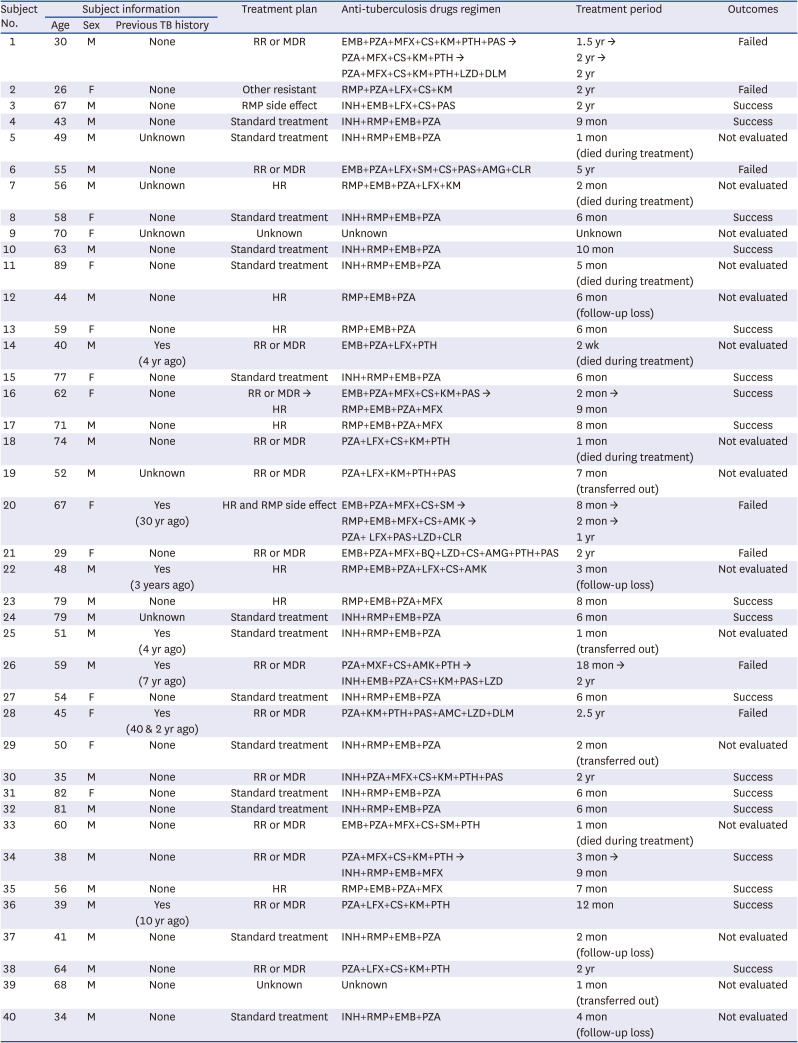
910 representing one of the largest obstacles to TB eradication. Resistant TB infection is caused by 1) direct transmission from other resistant TB patients and/or 2) selective pressure owing to incorrect drug use (such as incorrect drug selection and poor compliance). Therefore, the quick and accurate diagnosis of TB and drug resistance are key factors to determine the outcome of the fight against TB.
Recently, the importance of next-generation sequencing (NGS) has been emphasised in antimicrobial susceptibility testing, including in TB patients. This new technique can overcome the limitations of speed and accuracy of existing tests such as rapid molecular genotypic tests and conventional phenotypic tests. Thus, in 2018 the World Health Organization (WHO) published new guidelines concerning ‘The use of next-generation sequencing technologies for the detection of mutations associated with drug resistance in
Mycobacterium tuberculosis complex: technical guide’ to encourage the use of NGS.
211 NGS analysis can be divided into targeted sequencing and whole-genome sequencing (WGS). NGS based WGS is a method used to identify variants in the entire genome. This method is better suited to finding rare and novel mutations and obtaining information on several targets at once. For example, in addition to resistance, strain analysis is also possible, and it is very helpful for epidemiological investigations
111213; therefore, several governmental public health centres and large-scale medical centres in the United Kingdom, Italy, the United States, and Taiwan have tried using NGS based WGS for TB analysis.
14151617181920
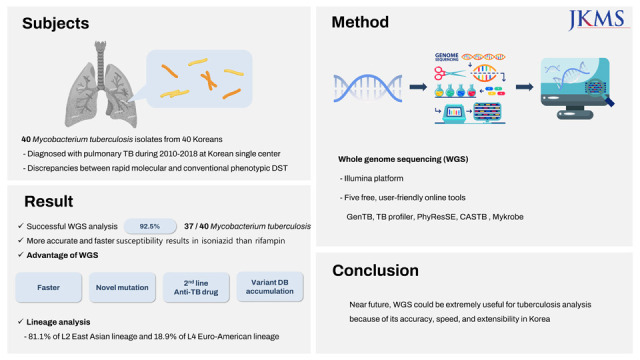
This study was designed to assess the usefulness of WGS for TB treatment in the clinical setting. We collected cases with INH- or RMP-related discrepancies between their rapid molecular and phenotypic drug susceptibility tests (DSTs) and reviewed the limitations of these two methods. In addition, we evaluated five user-friendly and free WGS analysis tools, GenTB, TB profiler, PhyResSE, CASTB, and Mykrobe, which were specified in the WHO guideline,
11 and whether these WGS tools could enhance the outcome of TB diagnosis and treatment.
DISCUSSION
This study was performed to evaluate the value that WGS adds to the commonly used protocols for TB DSTs in Korea. Our data revealed that WGS allows for the collection of more diverse data than the current methods used for this type of evaluation.
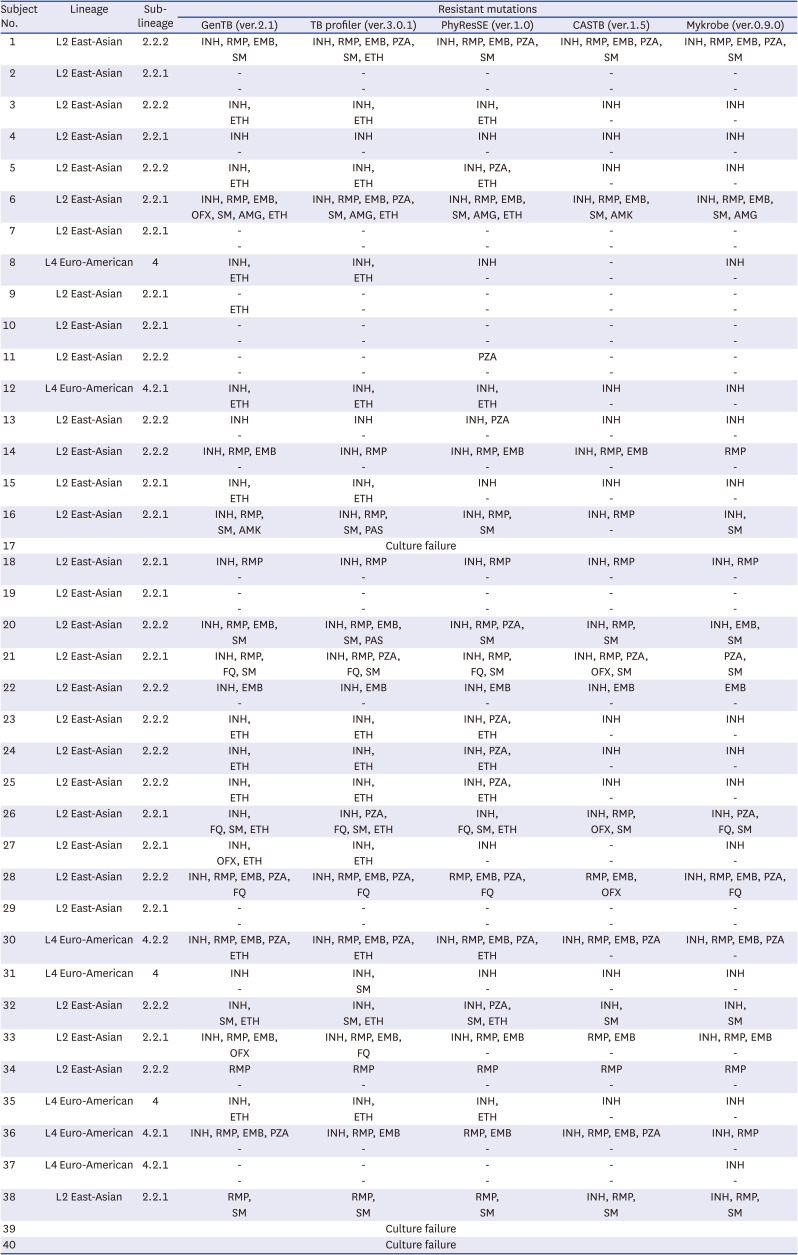
First, WGS allowed us to evaluate both DST and lineage distribution simultaneously. In the present study, the lineage distribution of the TB strains was consistent with previously reported data from East Asia,
1722 with the L2 East Asian lineage being dominant (70–90%) and the L4 lineage being less prevalent (~10%). Lineage analysis is useful for epidemiological purposes and can be used to confirm the chain of infection,
19 which is helpful for patient management, as specific lineages are known to be highly correlated with specific resistance characteristics. In particular, the Beijing lineage, which was predominant in our cohort, presents with a high mutation rate when infecting humans and has been frequently associated with drug resistance in various regions.
232425
Second, WGS can uncover novel and rare mutations that may be beyond the scope of the existing rapid molecular tests (line probe assays and commercial real-time polymerase chain reaction). Previous studies reported that WGS is not inferior to these tests, even demonstrating more accurate results in some datasets.
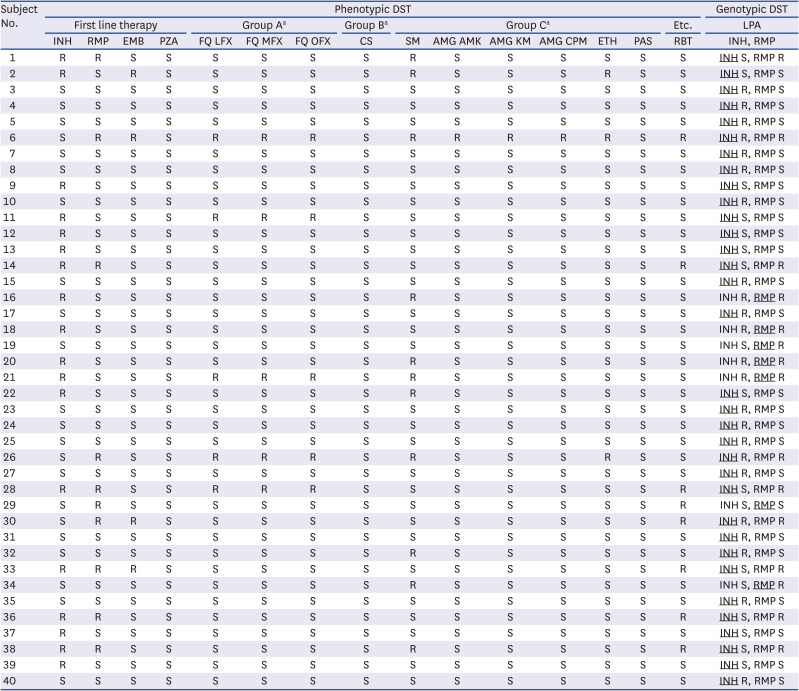
2627 In addition, our study clearly demonstrates the advantage of evaluating INH susceptibility/resistance using WGS as the concordance rate between the phenotypic DST and WGS (GenTB: 45.9% [17/37], TB profiler: 43.2% [16/37], PhyResSE: 40.5% [15/37], CASTB: 48.6% [18/37], and Mykrobe: 43.2% [16/37]) was much higher than that between the phenotypic DST and line probe assay (18.9% [7/37]). The reason for this large difference is likely associated with the fact that INH resistance-related mutations are scattered across a number of genes, including
katG,
fabG1-inhA,
oxyR-ahpC,
ndh,
kasA, and
iniB, meaning that commercial rapid molecular tests cannot cover them all. We also found novel mutations associated with INH in regions not covered by the line probe assay (
katG: p.Q50P, p.W191R, and c.649_650insG &
ahpC: c.-54C>T and c.-48G>A). Although the impact of these mutations remains ambiguous,
28 it is expected that their relationship with INH resistance can be revealed in future investigations.
For RMP, the concordance rate of the phenotypic DST and WGS, and phenotypic DST and line probe assays were very similar. This is likely because nearly all (95% or more) RMP-resistant strains harbour mutations within the 81 bp RMP-resistance-determining region (RRDR) in rpoB. Therefore, the line probe assay showed high sensitivity, despite only covering a short portion of this gene. However, it should be noted that approximately 5% of RMP-related mutations occur outside the RRDR sequence and may be missed when using rapid molecular tests.
Notably, we should be careful in interpreting the above INH and RMP concordance rates. This study was conducted only with about 40 strains showing discrepancies between their phenotypic DST and line probe assay. This sample selection bias significantly lowered the INH and RMP concordance rates. Thus, the above concordance rates were only used to explain the new mutations missing in the line probe assay, show detailed WGS information, and compare the five WGS software tools in the study. The fact that, for all 938 patients diagnosed with pulmonary TB in the Boramae Medical Center for 9 years, the INH concordance rate between the phenotypic DST and line probe assay was 95.6%, and RMP concordance rate between the phenotypic DST and line probe assay was 98.6%.
Finally, WGS is helpful when evaluating whether to use a drug that is not included in the commercial DST assays, such as quinolone or aminoglycoside. In addition, WGS examines the whole genome, allows for the storage of these data, and is at least 1 month faster than phenotypic DST. Hence, WGS is a promising tool for predicting resistance to new drugs, aiding in drug development, and managing TB in the future. It is clearly a useful tool for DST. In addition, since the five analysis tools evaluated in this study are free and very convenient to use, they can be easily accessed by clinicians and staff at general laboratories who are not highly trained bioinformatics experts. However, since each has different target antimicrobial agents and its own characteristic analysis mechanism, it is necessary to select the use after clearly understanding the strengths and weaknesses of each tool.
When we assessed the five tools with about 40 strains, there were no significant differences in the concordance of the drug-susceptible/resistant categories for these data from any of these online tools. Nevertheless, more importantly, each tool has specific its own mechanism such as bioinformatics pipeline, targeted drugs, relation genes, and upload system (
Supplementary Table 1). Therefore, since CASTB and Mykrobe were the least up-to-date and provided the least information, we would recommend that they be used only for auxiliary or confirmatory analyses. In contrast, GenTB was the most recently updated software and reported the most variants, including those that were not associated with antimicrobial resistance, making it a good screening tool but one that requires additional data trimming for clinical application. Thus, while GenTB demonstrates several significant advantages, the tool is not yet sophisticated enough on its own and should be supported by an additional evaluation methodology. Our experience suggests that this supporting tool should be PhyResSE, as it clearly displays the mapping results; the website would facilitate the evaluation and confirmation of the GenTB data.
While our data suggest that WGS has several advantages over current DST evaluations, the WGS technique has some limitations that must be overcome. It is very important to obtain high-quality whole-genome data for WGS. This can be complex in the case of Mycobacterium as these species produce a highly complex lipid cell layer that can interfere with genome extraction.
211 In addition, since live
M. tuberculosis has a high biohazard level, additional protection steps are recommended, making it difficult to complete genome extractions in a typical laboratory environment. Therefore, a simple, efficient, high-quality TB genome extraction method using direct samples, such as sputum, is needed to facilitate the widespread adoption of these technologies. In addition, considering the high cost and labour-intensive aspects, it is difficult to manage TB WGS in a general hospital laboratory with current technology. It would be effective that TB WGS to be processed in the central reference laboratory by collecting dozens of priority samples (acquired from TB reactivated patients, patients having severe anti-TB drug side effects, patients failing to first-line anti-drug, and so on) from several general laboratories. With remarkable advances in science and medicine, the technology speed is accelerating and the cost is falling, therefore, it is worth trying to introduce clinical use beyond research in general laboratories in near future.
Validated, standardised, and stable analysis tools, and database expansion are necessary to reach the full potential of high-throughput sequencing-based analyses. In the fields of genetic diseases and cancer, several NGS databases have been established with a high degree of investment to tackle various research questions. However, no such established databases exist for microorganisms. The coronavirus disease pandemic has highlighted the importance of microbial genome analysis, emphasizing the need for a centralised sequence database for such organisms, with many countries, institutions, and companies working toward its establishment. Therefore, in the post-coronavirus disease era, database management for various microorganisms, including
M. tuberculosis, should be systematised, substantially improving the potential of effective TB management.
2930
In summary, our data show that WGS is extremely useful for anti-TB DST. WGS identifies a much broader range of variants (especially for INH) compared with traditional rapid molecular techniques. In addition, WGS can confirm results more than 4 weeks earlier than phenotypic DST can, allowing for improved treatment planning and the prevention of TB transmission. Despite these advantages, additional research and development is needed to ensure that WGS can be adopted as a general laboratory diagnostic tool. However, considering the current speed of technological development and accumulated experience, we believe that these shortcomings will soon be overcome. In the near future, we expect that WGS will emerge as a commonplace technique that can be applied directly to culture-free specimens (such as sputum and bronchial wash) to determine the resistance of infectious agents to both first- and second-line anti-TB drugs within 1 week of specimen collection.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download