This article has been
cited by other articles in ScienceCentral.
PRESENTATION OF CASE 4
Dr. Sangsuk Choi: A 70-year-old man with no reported medical history visited our hospital due to gradually worsening painful swelling on the left distal forearm despite taking antibiotics for 2 months. The patient did not exhibit any other systemic symptoms such as fever. He was a fisherman in a small town on the coast of Jeollanam-do, without any recent history of invasive procedures or trauma that could induce or aggravate the lesion on his arm.
His vital signs were stable (body temperature, 37.0°C; blood pressure, 122/86 mmHg; heart rate, 74 beats per minute; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% in ambient air). The left distal forearm was swollen with erythema and purulent discharge, without any motor or sensory impairment. Physical examination of other body parts, such as the respiratory, gastrointestinal, and genitourinary systems, did not reveal anything in particular.
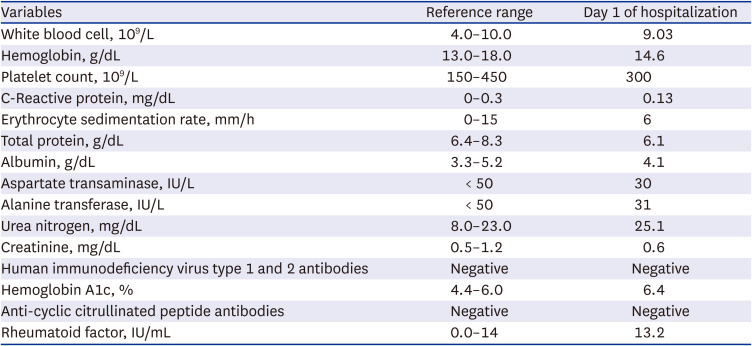
The patient was admitted to the department of orthopedic surgery to decide whether to have surgery. Pre-operative screening revealed normal levels of inflammatory markers, such as white blood cell count (9.03 × 10
3 cells/μL), erythrocyte sedimentation rate (6 mm/h), and C-reactive protein (0.13 mg/dL). Serologic test for antibodies against human immunodeficiency virus (HIV) was negative. The results of other laboratory tests are shown in
Table 1, and imaging studies were obtained.
Table 1
Laboratory data
|
Variables |
Reference range |
Day 1 of hospitalization |
|
White blood cell, 109/L |
4.0–10.0 |
9.03 |
|
Hemoglobin, g/dL |
13.0–18.0 |
14.6 |
|
Platelet count, 109/L |
150–450 |
300 |
|
C-Reactive protein, mg/dL |
0–0.3 |
0.13 |
|
Erythrocyte sedimentation rate, mm/h |
0–15 |
6 |
|
Total protein, g/dL |
6.4–8.3 |
6.1 |
|
Albumin, g/dL |
3.3–5.2 |
4.1 |
|
Aspartate transaminase, IU/L |
< 50 |
30 |
|
Alanine transferase, IU/L |
< 50 |
31 |
|
Urea nitrogen, mg/dL |
8.0–23.0 |
25.1 |
|
Creatinine, mg/dL |
0.5–1.2 |
0.6 |
|
Human immunodeficiency virus type 1 and 2 antibodies |
Negative |
Negative |
|
Hemoglobin A1c, % |
4.4–6.0 |
6.4 |
|
Anti-cyclic citrullinated peptide antibodies |
Negative |
Negative |
|
Rheumatoid factor, IU/mL |
0.0–14 |
13.2 |
IMAGING OBSERVATIONS
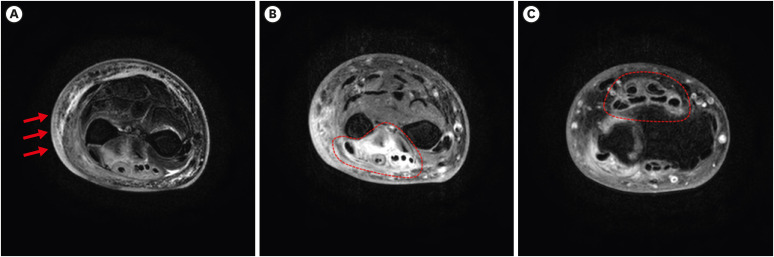
Dr. Seong-Heon Wie: Magnetic resonance imaging (MRI) of the left distal forearm revealed segmental skin swelling with faint enhancement, as well as some fluid collection in the subcutaneous fat layer, irregular fascial thickening with enhancement in the extensor and abductor pollicis longus muscles, and segmental remarkable synovial thickening with enhancement in the extensor and flexor muscles (
Fig. 1).
Fig. 1
Magnetic resonance imaging of the left distal forearm. (A) Magnetic resonance imaging showed segmental skin swelling with faint enhancement, some fluid collection in the subcutaneous fat layer. (B) Irregular fascial thickening with enhancement in extensor muscle, abductor pollicis longus muscles. (C) Segmentally remarkable synovial thickening with enhancement in extensor and flexor muscles.
CLINICAL IMPRESSION
Dr. Mi-Hee Kim: Chronic infectious arthritis, cellulitis, and myositis were considered as initial diagnoses based on physical examination and imaging findings.
DIFFERENTIAL DIAGNOSIS
Chronic infectious arthritis
Mycobacteria or fungi and other bacteria such as
Borrelia burgdorferi,
Tropheryma whipplei,
Treponema pallidum, and
Nocardia spp. can cause chronic infectious arthritis.
1 Histopathological and microbiological tests of fluid and tissue specimens can confirm the presence of pathogens such as mycobacteria, fungi, and bacteria. Arthritis caused by
B. burgdorferi,
T. whipplei, and
Nocardia spp. is often accompanied by systemic symptoms or signs such as fever, myalgia, fatigue, headache, cough, diarrhea, and enlarged lymph nodes. However, this patient neither had fever nor other systemic symptoms other than those of the left forearm described previously. The physical examination did not reveal any indicators except for findings of the left forearm. The result of the rapid plasma reagin test for syphilis was negative.
Systemic host factors in chronic infectious arthritis
On determining chronic infectious arthritis, it is important to evaluate systemic host factors such as diabetes mellitus, rheumatoid arthritis, and HIV infection.
2 The total score of the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria was 3, including 1 small joint (left wrist, 2 points) and ≥ 6-week symptom duration (1 point). The total score did not meet the minimum standard of the classification criteria for rheumatoid arthritis.
3 The serologic test for HIV was negative, and the hemoglobin A1C (HbA1c) test measured 6.40% (reference range, 4.4–6.0%) on day 1 of hospitalization. The patient was not diabetic earlier, and the level of HbA1c was identified as 5.40% after 3 months without requiring diabetes medication.
Social and local host risk factors
Social and local host risk factors could play important roles in chronic infectious arthritis, but local risk factors, such as direct trauma and recent surgery in the joints, and open fracture reduction, or social host risk factors, such as exposure to animal bites, alcohol abuse, and intravenous drug abuse were not present.
2 The patient’s career as a fisherman indicates that he was subjected to long periods of physical labor and repetitive movements.
INTRAOPERATIVE FINDINGS
Dr. Hyun Woo Park: Levofloxacin was administered as an empirical antibiotic. The patient then underwent surgical irrigation and debridement of the lesion for further diagnosis and treatment. The subcutaneous fat adhered to the 4th and 5th extensor compartments, and thickened inflammatory tenosynovium and tendon adhesion in the tenosynovium were observed. The extensor digiti minimi tendon was ruptured and retracted. Multiple fragments of tan-colored tissue were removed during surgery. A pathology report was obtained.
PATHOLOGICAL FINDINGS
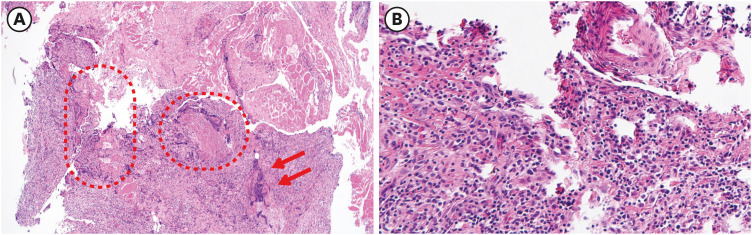
Dr. Mi-Hee Kim: Histological analysis of the tissues obtained from the operation showed acute and chronic inflammation and lymphohistiocytic infiltration with synovial regenerative changes (
Fig. 2).
Fig. 2
Histopathological analysis. (A) Synovial regenerative change (dotted line) and necrotic change with neutrophil infiltration (arrow) (×40, H&E stain) (B) Lymphohistiocytic infiltration on synovial tissues (×100, H&E stain).
H&E = hematoxylin-eosin.
Dr. Seong-Heon Wie: After discharge, the patient visited the outpatient department of infectious diseases. Oozing and swelling continued at the surgical site of the left forearm.
Bacterial and fungal cultures as well as a direct mycobacterial smear of the specimen from surgery yielded negative results. However, polymerase chain reaction (PCR) and acid-fast bacilli (AFB) culture to detect mycobacterium were positive for non-mycobacterium tuberculosis. The antibiotics were then changed to clarithromycin and doxycycline in response to this finding. In addition, microbial identification and antibiogram susceptibility tests of non-tuberculous mycobacteria (NTM) were performed.
MICROBIOLOGICAL TESTING
Dr. Soo-Young Kim: The NTM isolated from AFB culture was identified as Mycobacterium marinum or Mycobacterium ulcerans using the PCR-hybridization method, and the minimal inhibitory concentration (MIC) values of antibiotics for the mycobacteria were as follows: clarithromycin, ≤ 1 μg/mL; amikacin, ≤ 2 μg/mL; moxifloxacin, 0.25 μg/mL; linezolid, ≤ 1 μg/mL; ciprofloxacin, 1 μg/mL; doxycycline, 0.5 μg/mL; trimethoprim/sulfamethoxazole, 1/19 μg/mL; ethambutol, 8 μg/mL; rifampicin, ≤ 0.25 μg/mL; and rifabutin, 0.5 μg/mL. The isolated NTM pathogen was susceptible to clarithromycin and doxycycline.
MOLECULAR BIOLOGICAL TESTING
To identify the pathogen accurately, rpoB and hsp65 sequence analyses were performed using next-generation sequencing (NGS). The results for the rpoB and hsp65 sequences showed that the similarity values of M. marinum and M. shottsii were high: rpoB, 100.00% vs. 99.73%; hsp65, 100.00% vs. 99.85%. The percent similarities of both rpoB and hsp65 sequences of M. marinum were 100.00%, and the pathogen was identified as M. marinum.
GENERAL INTRODUCTION OF THE DISEASE MANAGEMENT
Dr. Mi-Hee Kim: The optimal treatment guideline for NTM infections is not completely established for most species and can be challenging to produce. Reportedly, infections due to
M. marinum are usually treated medically, and surgical management may be needed for deep tissue infections.
M. marinum is susceptible to clarithromycin, amikacin, rifampin, linezolid, ethambutol, trimethoprim/sulfamethoxazole, and combination therapy with at least two active drugs, including clarithromycin, rifampin, and/or ethambutol. The duration of treatment ranges from 2–6 months, depending on disease severity.
45
PROGNOSIS
Dr. Seong-Heon Wie: This patient was treated with two antibiotics, clarithromycin and doxycycline. One month after antibiotic administration was started, his pain was relieved, but a small amount of discharge was observed in the wound. After 4 months of antibiotic administration, his clinical condition, including wound discharge and pain, significantly improved.
DISCUSSION
Dr. Raeseok Lee: Were there any findings suggestive of NTM infection on history-taking and physical examination?
Dr. Mi-Hee Kim: The wound did not improve even after taking antibiotics for 2 months. Therefore, infection by atypical pathogens, including mycobacteria, should be considered first, followed by inflammatory diseases such as rheumatoid arthritis and ankylosing spondylitis.
Mycobacterial skin and soft tissue infections (SSTIs) should be considered when there is a history of environmental exposure, failure to respond to empirical antibiotic coverage for staphylococcal and streptococcal pathogens, and failure to grow pathogens on routine bacterial cultures. However, it can be difficult to diagnose because the clinical presentation varies from rashes, cellulitis, nodules, and abscesses to ulcer, and the lesion can be found in localized or disseminated forms.
6 Therefore, AFB culture or lesion biopsy is required to diagnose mycobacterial SSTIs. However, histopathologic evaluation had limitations in the further identification of mycobacterial species. Therefore, AFB culture should be performed.
Dr. Raeseok Lee: Is the patient’s occupation related to the M. marinum infection?
Dr. Seong-Heon Wie:
M. marinum is a waterborne mycobacterium that causes localized SSTIs following exposure to contaminated water or infected animals. Fishermen, fishery workers, and seafood handlers are vulnerable to this infection. It rarely infects deeper tissues and causes tenosynovitis, arthritis, or osteomyelitis.
45
Dr. Raeseok Lee: What other NTM species, except M. marinum, cause SSTI, and what are their clinical characteristics?
Dr. Mi-Hee Kim: NTM is ubiquitous in the soil and water niche of the environment and more than 190 species have been identified to date. The most common pathogens in SSTIs caused by NTM are
M. fortuitum,
M. chelonae,
M. abscessus,
M. marinum, and
M. ulcerans. SSTIs caused by
M. fortuitum,
M. chelonae, and
M. abscessus are usually transmitted via direct inoculation through plastic surgery, injections, body piercings, tattoos, acupuncture, or trauma. The clinical presentations of SSTIs caused by these NTM species are nonspecific and range from rashes, papules, plaques, nodules, cellulitis, and abscesses to ulcers.
M. ulcerans causes Buruli ulcer, which has been mostly reported in tropical areas, including Africa and Australia, and is not endemic to Korea. Buruli ulcers predominantly affect children in the lower extremities (> 55%).
M. ulcerans usually secretes the toxin mycolactone and causes a solitary, asymptomatic, and firm nodule, which is initially smaller than 5 cm in diameter, and then develops into necrotic ulcers larger than 15 cm. These ulcers are called Buruli ulcers.
47
M. shottsii also demonstrated high levels (99.73% and 99.85%) of
rpoB and
hsp65 sequence similarity in the results of the NGS analysis for NTM species identification in this case. It has been reported that
M. shottsii is a
Mycobacterium ulcerans/
Mycobacterium marinum clade (MuMC) member in the study of genomic analysis. However,
M. shottsii is mainly isolated from striped bass (
Morone saxatilis) and causes visceral and dermal mycobacteriosis in fishes; human diseases caused by
M. shottsii have not been reported yet.
8
In this case, the patient was microbiologically diagnosed with NTM infection, and M. ulcerans and M. marinum with similar genome sequences were reported as potential pathogens in the PCR-hybridization analysis. Finally, through NGS analyses of the rpoB and hsp65 sequences, the pathogen was identified as M. marinum.
ACKNOWLEDGMENTS
The Case Conference section is prepared from monthly case conference of Department of Internal Medicine, the Catholic University of Korea College of Medicine, Seoul, Korea.
References
1. Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia, PA, USA: Elsevier Health Sciences;2019.
2. Mathew AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol. 2014; 28(6):935–959. PMID:
26096095.
3. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford). 2012; 51(Suppl 6):vi5–vi9. PMID:
23221588.
4. Franco-Paredes C, Marcos LA, Henao-Martínez AF, Rodríguez-Morales AJ, Villamil-Gómez WE, Gotuzzo E, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018; 32(1):e00069-18. PMID:
30429139.
5. Wi YM. Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect Chemother. 2019; 51(3):245–255. PMID:
31583858.
6. Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014; 16(11):438. PMID:
25339245.
7. Chung J, Ince D, Ford BA, Wanat KA. Cutaneous infections due to nontuberculosis mycobacterium: recognition and management. Am J Clin Dermatol. 2018; 19(6):867–878. PMID:
30168084.
8. Gauthier DT, Doss JH, LaGatta M, Gupta T, Karls RK, Quinn FD. Genomic degeneration and reduction in the fish pathogen
Mycobacterium shottsii. Microbiol Spectr. 2022; 10(3):e0115821. PMID:
35579461.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download