Abstract
Purpose
Children with nephrotic syndrome may experience disease relapse or aggravation triggered by various viral infections. Limited studies on the clinical implications of the coronavirus disease 2019 (COVID-19) pandemic in children with nephrotic syndrome have been published worldwide. Therefore, this study aimed to investigate the effects of COVID-19 on the clinical course of nephrotic syndrome in children.
Methods
The medical records of 59 patients with idiopathic nephrotic syndrome who visited our hospital between February and June 2022 were retrospectively analyzed.
Results
Twenty of the total 59 patients with nephrotic syndrome were diagnosed with COVID-19 during the study period. The mean age at the time of the diagnosis of nephrotic syndrome and COVID-19 in all 20 patients was 4.6±3.5 and 8.9±3.9 years, respectively. Three patients (15%) were diagnosed with nephrotic syndrome relapse during COVID-19 and the relapse rate was similar to them without COVID-19 (20.5%, 8/39 patients). At the time of the COVID-19 diagnosis, fever (85%) and cough (40%) were the most common symptoms. After the diagnosis of COVID-19, all patients showed improvement with symptomatic treatment, including antipyretic analgesics and cold medicine. None of the critical patients required hospitalization or oral antiviral medications.
Conclusions
Despite the use of immunosuppressants, the clinical manifestations of COVID-19 in children with nephrotic syndrome were not severe and are expected to be similar to that in the general population. The relapse rate of nephrotic syndrome in children with COVID-19 was also not different from them without COVID-19.
Since its first outbreak in 2019, coronavirus disease 2019 (COVID-19) has spread rapidly worldwide, affecting millions of people differently. South Korea has also been heavily affected by COVID-19, with 23.6 million confirmed cases and 27,149 deaths reported as of September 5, 2022. Among the total number of confirmed cases, 5.67 million cases were reported in children aged <19 years, accounting for 24% of the total cases, with 46 deaths, indicating that the severity of COVID-19 in pediatric patients was significantly lower than that in adults [1]. Several reports have described de novo kidney involvement and aggravation of underlying renal diseases due to COVID-19 or vaccination in adult patients [2-6]. Recently, cases of renal complications due to COVID-19 or vaccination have been reported in pediatric patients [7-9]; however, the clinical significance of COVID-19 in pediatric patients with kidney disease is unclear. In addition, although nephrotic syndrome relapse is closely related to various viral infections, there are limited studies on the clinical implications of the COVID-19 pandemic in pediatric patients with nephrotic syndrome. Therefore, this study aimed to investigate the effect of COVID-19 on the clinical course of nephrotic syndrome in children.
The medical records of pediatric patients with idiopathic nephrotic syndrome who visited our hospital between February and June 2022 were retrospectively analyzed. Patients aged <1 year and >19 years and those with inadequate medical records were excluded. Finally, total 59 patients (20 pediatric patients diagnosed with COVID-19 and 39 pediatric patients who did not have COVID-19) were included in the study. Relapse of the nephrotic syndrome within 2 weeks of COVID-19 was defined as a relapse caused by COVID-19. The diagnosis of COVID-19 was confirmed using real-time polymerase chain reaction or rapid antigen testing performed at a public health center hospital. Student t-test was performed to evaluate continuous variables. Statistical significance was set at a P-value of <0.05. All statistical analyses were conducted using the R software, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
The baseline characteristics of 59 nephrotic syndrome patients who visited our hospital during the study period are as follows: mean age at the study period, 9.3±3.7 years; mean age at diagnosis of nephrotic syndrome, 4.8±3.1 years; male, 79.7%; steroid-sensitive nephrotic syndrome, 81.4% (Table 1).
Twenty of the total 59 patients with nephrotic syndrome were diagnosed with COVID-19 during the study period (Table 2). The mean age at the time of the diagnosis of nephrotic syndrome of 20 patients confirmed with COVID-19 during the study period was 4.6±3.5 years. Their mean age at the time of COVID-19 diagnosis was 8.9±3.9 years, and the mean period from the diagnosis of nephrotic syndrome to that of COVID-19 was 4.6 years. In study population, most COVID-19 cases occurred in April 2022, similar to the general trend of COVID-19 cases in South Korea (Fig. 1). The ratio of patients with steroid-sensitive nephrotic syndrome to those with steroid-resistant nephrotic syndrome was 14:6. All patients visited the hospital for regular follow-up within a month before contracting COVID-19, and the urinalysis results at that time showed no proteinuria. The drugs used for the treatment of nephrotic syndrome during COVID-19 included patients receiving corticosteroid therapy alone (n=5), cyclosporine therapy alone (n=4), corticosteroid and cyclosporine combination therapy (n=3), rituximab therapy within 1 year (n=3), tacrolimus therapy alone (n=1), and corticosteroid and tacrolimus combination therapy (n=1). The mean daily dose for patients receiving steroids was 0.7±0.6 mg/kg. The mean dose of tacrolimus was 0.05±0.01 mg/kg/day, while that of cyclosporine was 5.3±0.4 mg/kg/day. The rate of immunosuppressant use was significantly lower in patients without COVID-19 than in patients with COVID-19 (P<0.01). However, the other baseline characteristics were similar between the groups. At the time of COVID-19 diagnosis, fever (85%) and cough (40%) were the most common symptoms. Other symptoms, such as abdominal pain, diarrhea, and headache were also present. Two of the 20 patients (10%) diagnosed with COVID-19 were vaccinated before diagnosis. Three of the 20 patients with COVID-19 were treated with CD20 monoclonal antibody (rituximab) within 1 year before being diagnosed with COVID-19. In all of them, the administration effect was confirmed by measuring complete depletion of B cells (<1% of lymphocytes) immediately after CD20 monoclonal antibody (rituximab) therapy. However, because they were diagnosed with COVID-19 at the local medical centers and none of them experienced nephrotic syndrome relapse or severe COVID-19 clinical manifestations, they did not visit our hospital and recovered without antiviral medications. After the diagnosis of COVID-19, all 20 patients experienced improvement with symptomatic treatment, including antipyretic analgesics and cold medicine. None of the patients developed critical conditions requiring hospitalization or the administration of oral antiviral medications such as Paxlovid.
Three (15%) of the 20 patients with COVID-19 were diagnosed with nephrotic syndrome relapse while COVID-19 was ongoing (Table 3). Patients with nephrotic syndrome who visited our hospital during the study period but did not contract COVID-19 had a relapse rate of 20.5% (eight of 39 patients). All three patients diagnosed with nephrotic syndrome relapse complained of fever and abdominal pain during COVID-19, and none of the three patients diagnosed with nephrotic syndrome relapse during COVID-19 were vaccinated. One of the three patients diagnosed with nephrotic syndrome relapse during COVID-19 was not receiving immunosuppressants. Of the other two patients, one received steroids at a dose of 0.4 mg/kg/day, and the other received cyclosporine at a dose of 5.5 mg/kg/day. In these two patients, the dose of immunosuppressants was not higher than that in patients without relapse during COVID-19. All patients who relapsed during COVID-19 responded well to corticosteroid treatment at the time of relapse.
The number of COVID-19 cases in the pediatric age group was low during the early stages of the pandemic, but it gradually increased as the unprecedented COVID-19 pandemic continued to spread worldwide [10]. A systematic review published in Pediatric Pulmonology in 2020 reported that >50% of pediatric patients with COVID-19 complained of asymptomatic or mild symptoms, and approximately 1% of patients complained of critical clinical symptoms [11]. The most common symptoms were fever, cough, nasal symptoms, diarrhea, and nausea/vomiting [11-13]. COVID-19 may also cause various pulmonary, gastrointestinal, cardiovascular, neurological, and renal complications [11,14-16]. In addition to possibly increasing risks of new kidney disease, COVID-19 may also worsen the course of patients with underlying kidney disease. Acute kidney injury is an important complication of COVID-19, and several cases of acute tubulointerstitial nephritis and acute glomerulonephritis in pediatric patients have been reported [4,5]. Shah et al. [17] first reported on a new-onset nephrotic syndrome associated with COVID-19 in an 8-year-old boy, followed by Alvarado et al. [18], who reported on a case of COVID-19 associated with the onset of nephrotic syndrome in a 15-year-old boy. COVID-19 has various effects on the clinical courses of patients with underlying diseases. Recently, a number of reports related to nephrotic syndrome relapse have been published, with some focusing on pediatric patients. Moreover, the incidence of nephrotic syndrome relapse is increasing in children with upper respiratory tract infections. According to previous studies, at least 50% of nephrotic syndrome relapses are caused by upper respiratory tract infections [19,20]. The mechanism by which infection leads to nephrotic syndrome relapse is unclear, but it may be related to a non-specific host response, such as cytokine release in response to infection [20]. It has been hypothesized that infection-induced nephrotic syndrome relapse can be caused by podocytopathy based on the concepts of immune dysregulation and increased glomerular permeability [21]. In pediatric patients, the symptoms of COVID-19 vary, and the infection severity is not as high as that reported in adult patients [12,13]. The pediatric patients with nephrotic syndrome included in this study also had various symptoms, and all complained of only mild symptoms that improved spontaneously without hospital treatment. An original article published by Crane et al. [22] and a systematic review published by Morello et al. [23] found that pediatric nephrotic syndrome did not increase the severity of COVID-19 and that the relapse rate of nephrotic syndrome triggered by COVID-19 was low. In the present study, only three of the 20 patients (15%) were diagnosed with nephrotic syndrome relapse due to COVID-19, all of whom responded well to steroid treatment. The long-term use of immunosuppressive agents in pediatric patients with nephrotic syndrome did not adversely affect the severity of COVID-19, consistent with findings of previous studies [23-25]. The association between rituximab and COVID-19, which has a significant effect on immune response, also showed similar results. Recently, the frequency of rituximab administration is increasing for the treatment of pediatric nephrotic syndrome since the public health insurance benefits were applied in South Korea. Although there has been a substantial debate about the administration of rituximab in children concerning infection associated with the COVID-19 pandemic, several studies have demonstrated that rituximab has no significant effect on the incidence, clinical severity, or antibody production in pediatric patients with COVID-19 [26,27]. In the present study, four patients received rituximab treatment 4 to 9 months before the study period, none of whom were diagnosed with nephrotic syndrome relapse or other severe complications of COVID-19. Therefore, pediatric patients with nephrotic syndrome requiring immunosuppressive agents such as rituximab can receive treatment, even in the special situation of the COVID-19 pandemic.
However, this study has some limitations. First, the small number of included patients owing to the short observation period. Therefore, information on later complications could not be confirmed. Second, it cannot be ruled out that patients who were not diagnosed with COVID-19 during the study period had asymptomatic COVID-19. However, to the best of our knowledge, this study is the first study to report the clinical features of COVID-19 in Korean children with nephrotic syndrome. These findings provide valuable evidence for determining the clinical prognosis of pediatric patients with nephrotic syndrome diagnosed with COVID-19.
In conclusion, the clinical manifestations of COVID-19 in children with nephrotic syndrome were not severe in spite of receiving the immunosuppressant medications and are expected to be similar to that in the general population. Further, the relapse rate of nephrotic syndrome in children with COVID-19 was not different from them without COVID-19.
Notes
Ethical statements
This retrospective study was conducted in accordance with the principles of the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of Kyungpook National University Hospital (IRB No. 2022-09-006), which waived the need for written informed consent.
References
1. Korea Disease Control and Prevention Agency. Coronavirus (COVID-19) [Internet]. Cheongju: Korea Disease Control and Prevention Agency;c2022. [cited 2022 Sep 5]. Available from: http://ncov.mohw.go.kr/en/.
2. Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021; 78:142–5.

3. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021; 6:2969–78.

4. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020; 31:1959–68.

5. Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020; 31:1380–3.

6. Bomback AS, Kudose S, D’Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021; 78:477–80.

7. Bjornstad EC, Krallman KA, Askenazi D, Zappitelli M, Goldstein SL, Basu RK, et al. Preliminary assessment of acute kidney injury in critically ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol. 2021; 16:446–8.
8. Serafinelli J, Mastrangelo A, Morello W, Cerioni VF, Salim A, Nebuloni M, et al. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr Nephrol. 2021; 36:3789–93.

9. Hanna C, Herrera Hernandez LP, Bu L, Kizilbash S, Najera L, Rheault MN, et al. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021; 100:705–6.

10. Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19: COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1081–8.
11. de Souza TH, Nadal JA, Nogueira RJ, Pereira RM, Brandao MB. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol. 2020; 55:1892–9.

12. Badal S, Thapa Bajgain K, Badal S, Thapa R, Bajgain BB, Santana MJ. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J Clin Virol. 2021; 135:104715.

13. Parcha V, Booker KS, Kalra R, Kuranz S, Berra L, Arora G, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021; 11:10231.

14. Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021; 143:21–32.

15. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020; 26:1017–32.

16. Basalely A, Gurusinghe S, Schneider J, Shah SS, Siegel LB, Pollack G, et al. Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int. 2021; 100:138–45.

17. Shah SA, Carter HP. New-onset nephrotic syndrome in a child associated with COVID-19 infection. Front Pediatr. 2020; 8:471.

18. Alvarado A, Franceschi G, Resplandor E, Sumba J, Orta N. COVID-19 associated with onset nephrotic syndrome in a pediatric patient: coincidence or related conditions? Pediatr Nephrol. 2021; 36:205–7.

19. MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. 1986; 108:378–82.

20. Uwaezuoke SN. Steroid-sensitive nephrotic syndrome in children: triggers of relapse and evolving hypotheses on pathogenesis. Ital J Pediatr. 2015; 41:19.

21. Zhang H, Wang Z, Dong L, Guo Y, Wu J, Zhai S. New insight into the pathogenesis of minimal change nephrotic syndrome: role of the persistence of respiratory tract virus in immune disorders. Autoimmun Rev. 2016; 15:632–7.

22. Crane C, Bakhoum C, Ingulli E. Rates of idiopathic childhood nephrotic syndrome relapse are lower during the COVID-19 pandemic. Pediatr Nephrol. 2022; 37:2679–85.

23. Morello W, Vianello FA, Proverbio E, Peruzzi L, Pasini A, Montini G. COVID-19 and idiopathic nephrotic syndrome in children: systematic review of the literature and recommendations from a highly affected area. Pediatr Nephrol. 2022; 37:757–64.

24. Angeletti A, Drovandi S, Sanguineri F, Santaniello M, Ferrando G, Forno R, et al. COVID-19 in children with nephrotic syndrome on anti-CD20 chronic immunosuppression. Clin J Am Soc Nephrol. 2020; 15:1494–5.

25. Marlais M, Wlodkowski T, Al-Akash S, Ananin P, Bandi VK, Baudouin V, et al. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child. 2020; 106:798–801.

Fig. 1.
Comparison of trends in the cumulative number of confirmed coronavirus disease 2019 (COVID-19) cases in South Korea and our study subjects by month. Data from: Korea Disease Control and Prevention Agency (September 5, 2022) [1].
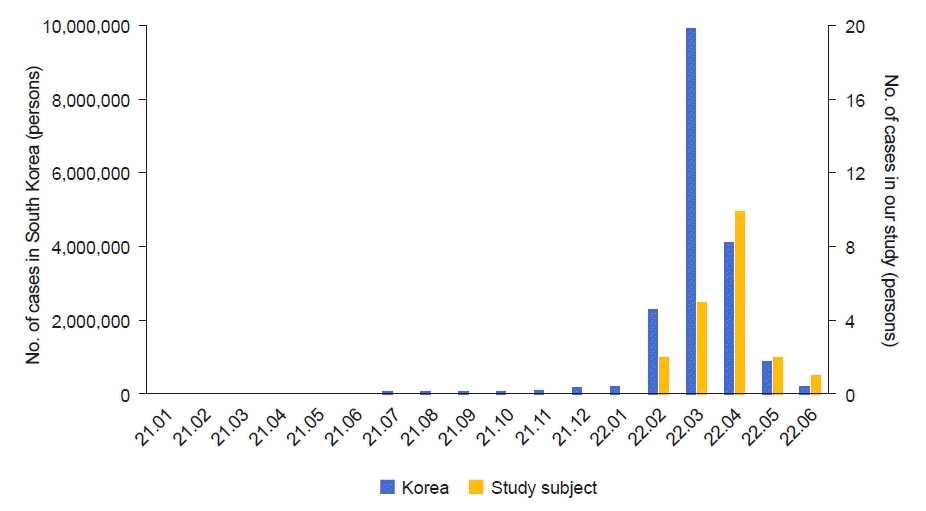
Table 1.
Baseline characteristics of patients with nephrotic syndrome
Table 2.
Comparison of clinical characteristics between the patients with and without COVID-19
Table 3.
Clinical characteristics of three patients diagnosed with nephrotic syndrome relapse during the COVID-19 illness




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download