1. Koh SB. The ideal strategies of chemotherapy for the treatment of cervical cancer. Kosin Med J. 2018; 33:283–8.

2. Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977; 269:518–21.

3. Mann WJ. Surgical management of radiation enteropathy. Surg Clin North Am. 1991; 71:977–90.

4. Zimmermann FB, Feldmann HJ. Radiation proctitis: clinical and pathological manifestations, therapy and prophylaxis of acute and late injurious effects of radiation on the rectal mucosa. Strahlenther Onkol. 1998; 174 Suppl 3:85–9.
5. Naito Y, Yoshikawa T. Rebamipide: a gastrointestinal protective drug with pleiotropic activities. Expert Rev Gastroenterol Hepatol. 2010; 4:261–70.

6. Kochhar R, Sriram PV, Sharma SC, Goel RC, Patel F. Natural history of late radiation proctosigmoiditis treated with topical sucralfate suspension. Dig Dis Sci. 1999; 44:973–8.
7. Livstone EM, Hersh T, Spiro HM, Floch MH. The gastrointestinal microflora of irradiated mice. II. Effect of oral antibiotic administration on the colonic flora and survival of adult mice. Yale J Biol Med. 1970; 42:448–54.
8. Ojetti V, Lauritano EC, Barbaro F, Migneco A, Ainora ME, Fontana L, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol. 2009; 5:675–82.

9. Kang G, Kim SE. How to write an original article in medicine and medical science. Kosin Med J. 2022; 37:96–101.

10. Kim DJ, Kil SY, Son J, Lee HS. How to conduct well-designed clinical research. Kosin Med J. 2022; 37:187–91.

11. Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983; 11:1475–89.

12. D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999; 342(Pt 2):249–68.

13. Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008; 83:804–16.

14. Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, et al. Extracellular NAMPT promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008; 283:34833–43.

15. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007; 404:1–13.

16. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23:2369–80.

17. Leibiger IB, Berggren PO. Sirt1: a metabolic master switch that modulates lifespan. Nat Med. 2006; 12:34–6.

18. Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004; 18:1272–82.

19. Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011; 21:146–58.

20. Linard C, Marquette C, Clarencon D, Galonnier M, Mathieu J, Pennequin A, et al. Acute ileal inflammatory cytokine response induced by irradiation is modulated by subdiaphragmatic vagotomy. J Neuroimmunol. 2005; 168:83–95.

21. Ostrau C, Hulsenbeck J, Herzog M, Schad A, Torzewski M, Lackner KJ, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009; 92:492–9.

22. Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010; 285:1283–95.

23. Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003; 2:717–26.

24. Van Deventer SJ. Tumour necrosis factor and Crohn's disease. Gut. 1997; 40:443–8.

25. Sen A, Paine SK, Chowdhury IH, Mukherjee A, Choudhuri S, Saha A, et al. Impact of interleukin-6 promoter polymorphism and serum interleukin-6 level on the acute inflammation and neovascularization stages of patients with Eales’ disease. Mol Vis. 2011; 17:2552–63.
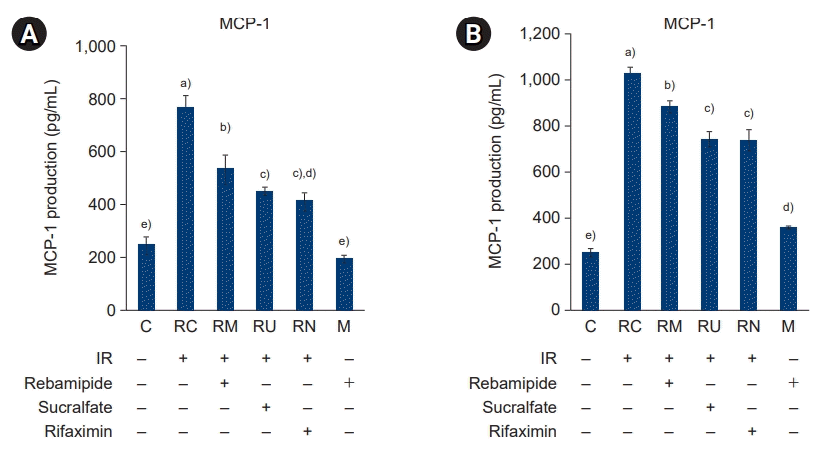
26. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009; 29:313–26.
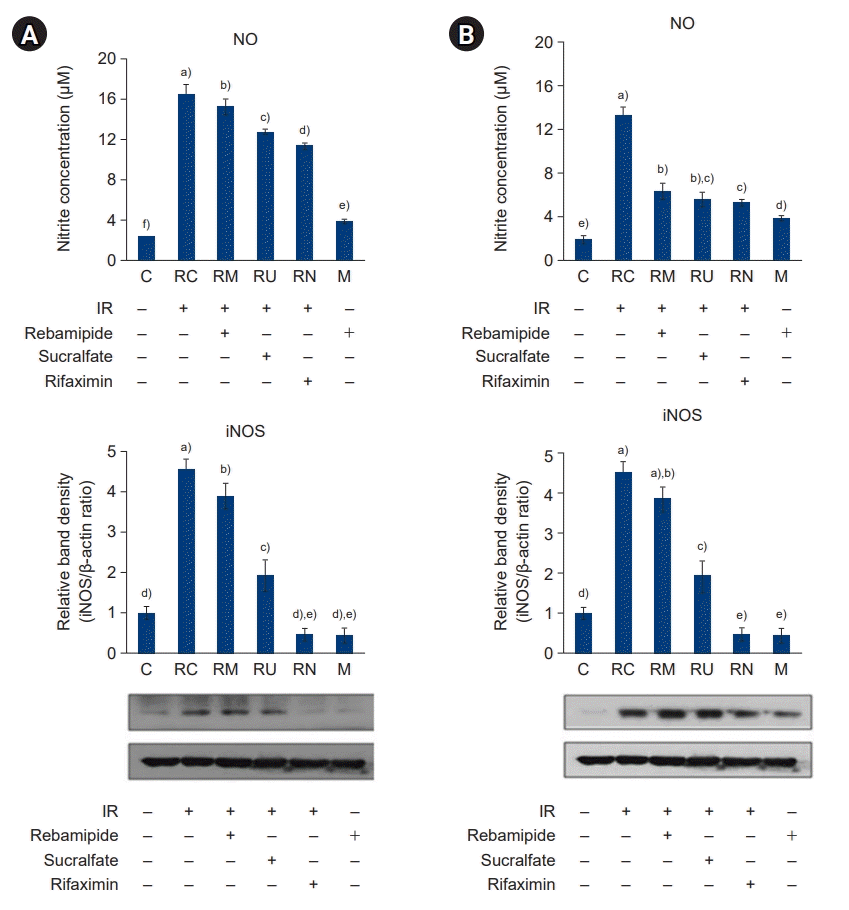
27. Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995; 16:128–30.

28. Kolli VK, Abraham P, Rabi S. Methotrexate-induced nitrosative stress may play a critical role in small intestinal damage in the rat. Arch Toxicol. 2008; 82:763–70.

29. Courtois F, Seidman EG, Delvin E, Asselin C, Bernotti S, Ledoux M, et al. Membrane peroxidation by lipopolysaccharide and iron-ascorbate adversely affects Caco-2 cell function: beneficial role of butyric acid. Am J Clin Nutr. 2003; 77:744–50.

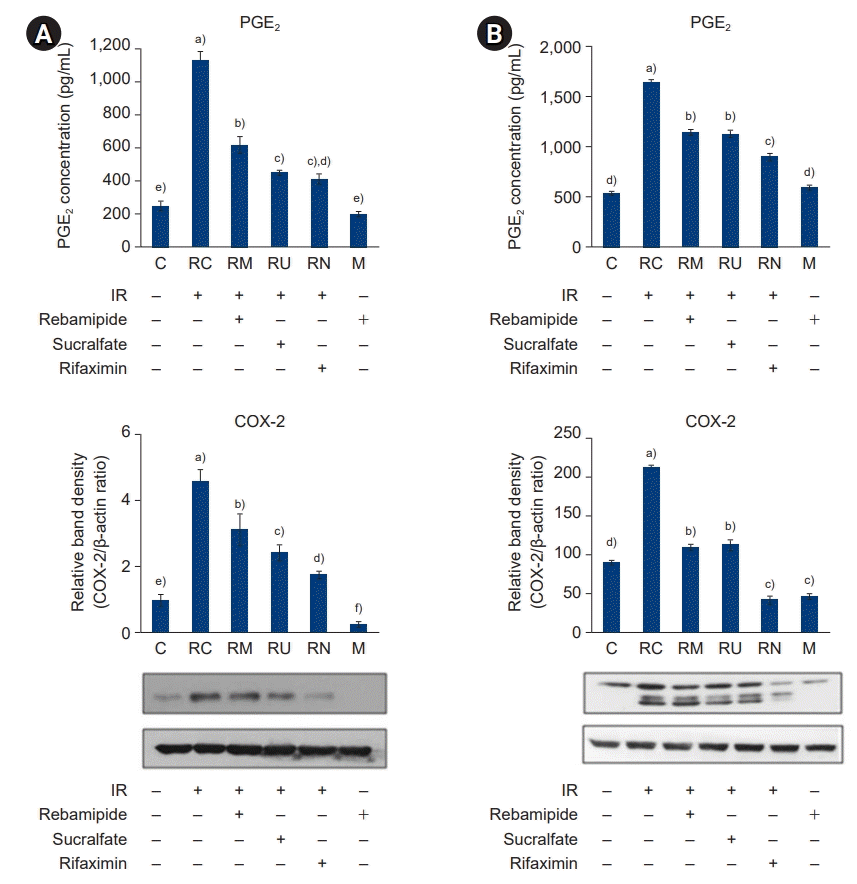
30. Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993; 268:9049–54.

31. Brzozowski T, Konturek PC, Konturek SJ, Brzozowska I, Pawlik T. Role of prostaglandins in gastroprotection and gastric adaptation. J Physiol Pharmacol. 2005; 56 Suppl 5:33–55.
32. Muller-Decker K, Furstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007; 46:705–10.

33. Gretzer B, Ehrlich K, Maricic N, Lambrecht N, Respondek M, Peskar BM. Selective cyclo-oxygenase-2 inhibitors and their influence on the protective effect of a mild irritant in the rat stomach. Br J Pharmacol. 1998; 123:927–35.

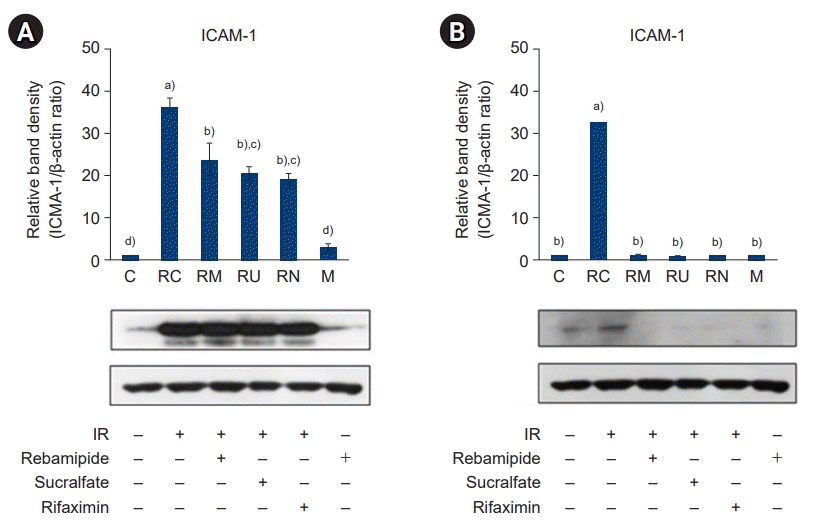
34. Molla M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, et al. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2003; 57:264–73.

35. Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999; 284:339–43.

36. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001; 292:727–30.

37. Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006; 311:847–51.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download