Abstract
Background
Pancreatic cystic lesions (PCLs) are occasionally found in solid organ transplant (SOT) recipients. In such recipients, the risk of cancer is increased due to immunosuppressive therapy. This study investigated the prevalence of PCLs and described their clinical course in immunosuppressed patients following SOT.
Methods
The presence of PCLs in a retrospective cohort of 805 consecutive SOT recipients from 2009 to 2019 was examined. The characteristics of PCLs were compared using initial and follow-up imaging, where available. These results were compared to an age- and sex-matched immunocompetent control group monitored for at least 12 months.
Results
PCLs were present in 15 of 805 SOT patients (seven liver and eight kidney transplantations). The median diameter of the largest lesion was 20 mm (range, 0.2–60 mm) and 60% of lesions were benign. During follow-up imaging, the cyst size remained stable in 46.7%, increased in 13.3%, and decreased in 40.0% of the SOT group. Significantly more of the SOT patients showed PCL size reductions (P=0.007). Among SOT patients diagnosed with intraductal papillary mucinous neoplasms (6/15), worrisome features were noted in one patient at the time of cyst diagnosis. Differences in the development of worrisome features between the study and control groups were not statistically significant.
Due to the high quality of cross-sectional imaging tools, incidental pancreatic cystic lesions (PCLs) are now diagnosed more frequently [1]. PCLs are detected in 2%–45% of the general population [2-5], with PCL classification and management differing by type of lesion [6-9] (Fig. 1). Due to the high rate of adverse events, especially the spread of malignancy, cytology brush or endoscopic ultrasound-guided fine-needle aspiration is not recommended for PCL. The diagnosis and evaluation of PCLs, therefore, still depend on imaging studies [10-13].
The histologic classification of PCLs includes benign, premalignant, and malignant. For premalignant or malignant PCL lesions—including intraductal papillary mucinous neoplasms (IPMN), mucinous cystic neoplasms (MCN), serous cystic neoplasms (SCN), solid pseudopapillary neoplasms, and cystic neuroendocrine tumors—appropriate imaging follow-up and precise evaluation of worrisome features (WFs) are needed. MCN and IPMN, which have features associated with a high risk for malignancy, and symptomatic PCLs (i.e., lesions causing gastrointestinal symptoms or jaundice due to effects of the mass) should be considered for surgical resection [1,14], albeit using slightly different surgical guidelines [8,9].
Patients undergoing solid organ transplantation (SOT) receive immunosuppressant drugs, and several epidemiological studies have demonstrated an increased incidence of various cancers among transplant recipients due to poor immune control of known oncogenic infections, loss of immune surveillance, or carcinogenic effects of immunosuppressive medications [15-17].
Although there have been reports on the malignancy potential of PCLs in SOT patients in Western countries, this appears to be the first report in Asia on this topic [15,18,19]. This study investigated the prevalence of PCLs and described their clinical course in immunosuppressed patients following SOT. Specifically, this study focused on whether immunosuppressed post-SOT patients had more frequent malignant transformations of PCLs than non-SOT patients diagnosed with PCLs.
This retrospective study conformed with the ethical guidelines of the Declaration of Helsinki and was approved by Institutional Review Board of Korea University College of Medicine (IRB No. 2014AN0285). Informed consent was waived by the Institutional Review Board.
This study retrospectively reviewed medical records and imaging studies of patients who received SOT and were also diagnosed with PCLs (the SOT group) at the Korea University Medical Center from 2009 to 2019. PCL diagnosis was confirmed by a manual review of imaging. Patients who had previously been diagnosed with pancreatic cancer, had imaging findings of high-risk stigmata (HRS) or WFs, had a history of chronic pancreatitis (because PCLs in these patients are more likely to be pseudocysts than cystic neoplasms), or were under age 19 were excluded. The included patients were compared with an immunocompetent control group of patients diagnosed with PCLs who did not undergo SOT and were monitored for at least 12 months. Age and sex were matched between the two groups.
Almost all SOT patients received tacrolimus for immunosuppression (Table 1). At our center, all patients receive a triple therapy for immunosuppression following transplantation, including a calcineurin inhibitor, mycophenolate, and steroids, with the steroids tapered down over 3 months. In the study group, there was one patient who also received sirolimus following liver transplantation (LT).
Computed tomography or magnetic resonance images of both preoperative workups and postoperative follow-ups for all SOT patients were reviewed to identify PCLs, which were diagnosed by abdominal radiologists. An imaging diagnosis of SCN was made for lesions composed of a cluster of numerous small cysts with or without a central scar, calcified or noncalcified [15]. Lesions were classified as MCN when a unilocular cyst of up to 3 cm, with or without mild septation, mural thickening, or enhancement, was seen without communication with the pancreatic duct [15]. A diagnosis of branch duct type IPMN (BD-IPMN) indicated the presence of at least one pancreatic cyst greater than 5 mm that clearly communicated with pancreatic ducts via a non-dilated (<5 mm) main pancreatic duct (MPD) [1,15]. MPD involvement was considered as probable if the duct’s diameter was greater than 5 mm without duct obstruction [1,15].
The size of PCLs was defined as the longest axial diameter of the largest PCL. The images compared were those taken immediately before the operation in the control group and the earliest images taken following transplantation in the SOT group, who were being monitored for PCLs. HRS and WFs were defined by the 2017 Fukuoka Consensus Guidelines [8]. HRS were considered highly likely to be malignancies, and lesions with HRS were recommended for resection. WFs were considered indicators of potential malignancy. HRS included jaundice, enhancing mural nodules of at least 5 mm, and MPD dilatation of at least 10 mm. Cysts of at least 3 cm, enhancing mural nodules less than 5 mm, thickened or enhancing cyst walls, main duct size of 5–9 mm, lymphadenopathy, an increased serum level of CA-19-9, and a cyst growth rate of at least 5 mm in 2 years were among the WFs. All patients diagnosed with PCL in the study group were followed up after transplantation.
A descriptive analysis of cross-sectional imaging was performed to determine the prevalence of PCLs in our SOT group. The prevalence of cysts by age, sex, and type of SOT was examined. All data was analyzed by the Student t-test or chi-square test as appropriate, using IBM SPSS ver. 28 (IBM Corp., Armonk, NY, USA). A P-value less than 0.05 was considered statistically significant.
The total number of SOT patients in this study was 805 (liver, 237; kidney, 568). Table 1 presents the initial patient findings. The SOT study group included 15 PCL patients, while there were 60 PCL patients in the control group. The lesion prevalence was 1.86% (15/805) in the study group, with six patients diagnosed with PCLs before transplantation, and nine after. Two-thirds of the patients diagnosed with PCLs in the SOT group were male, and the most commonly used immunosuppressant was tacrolimus.
In both groups, IPMN was the most common type of PCL, and most of the PCLs were single lesions (Table 2). The locations of PCLs were different between the two groups. In the SOT group, 60% (9/15) of PCLs were in the pancreas tail and 40% (6/15) were located in the pancreas head. In the control group, 33.3% (20/60), 28.3% (17/60), and 38.3% (23/60) of PCLs were in the pancreas head, body, and tail, respectively. A higher proportion of lesions, therefore, was in the pancreas tail in the SOT group (Table 2). Median diameters of the largest lesions were 2.40 cm (range, 0.2–60 mm) in the SOT group and 2.01 cm (range, 0.2–60 mm) in the control group.
The median follow-up duration was 56.4 and 41.73 months in the study and control groups, respectively (Table 2). In follow-up imaging studies, the size of cysts remained stable in 46.7%, increased in 13.3%, and decreased in 40.0% of the study group. There were therefore significantly more patients who showed PCL size decreases in the SOT group (Table 2). Among the patients diagnosed with IPMN (6/15), WFs were noted in one patient at the time of diagnosis. That patient received only conservative management due to the patient’s multiple comorbidities. There were no significant differences in the development of WFs between the study and control groups (Table 2).
This study examined PCLs in patients who underwent SOT, with the goal of investigating the potential effects of immunosuppressant administration on malignant transformation. Most of the PCLs in our SOT group did not change during follow-up. Additionally, WFs were found in only two study group patients (MPD dilatation) and four control group patients (mural nodule and MPD dilatation). There were no significant differences in worrisome IPMN features between the study and control groups. This suggests that for PCL without WFs, liver and kidney transplantation patients can be treated conservatively (i.e., without surgical intervention), with only serial imaging follow-up.
Vidhyarkorn et al. [15] reviewed 1,778 LT patients, found 70 patients with small incidental PCLs and observed the long-term outcomes of these lesions. Only one patient was identified as having mixed acinar-neuroendocrine carcinoma after 9 years. Even though the average size and number of PCLs increased by 4.5 mm and 1.4 respectively during the follow-up period, the majority of incidental PCLs in the LT patients were stable and non-malignant despite immunosuppression. In our study, the size of the cysts remained stable in most patients. In fact, a significantly higher percentage of SOT patients had PCL size decreases, a finding we are not able to explain.
Liu et al. [18] reported a PCL prevalence of 6.1% (53/872) in their cohort of Australian LT patients. Two patients were confirmed as MCN (3.7% of PCLs) and underwent surgical resection without operative complications. As in our study, the most common PCL was IPMN, and most lesions had a benign course during the follow-up period [18].
Lennon et al. [19] explored whether LT recipients with BD-IPMNs were at higher risk of developing high-risk features than patients with BD-IPMNs who did not receive a transplant. Twenty-three LT patients with BD-IPMN were compared with 274 control patients. The median length of follow-up was 53.7 and 24.0 months in LT and control groups, respectively. Four patients in the study group (17.4%) and 45 patients in the control group (16.4%) developed high-risk features. There was, therefore, no statistically significant difference in the risk of developing high-risk features between the two groups (P=0.99). Multivariate analysis also showed that the malignant progression of BD-IPMNs was associated with age at diagnosis, but not with LT [19]. They concluded that there was no statistically significant difference in the risk of developing high-risk features between the LT and the control groups [19]. Although multivariate analysis was not possible in our study due to the small cohort, the results are similar in suggesting that immunosuppression following SOT does not accelerate malignancy in PCLs. This supports conservative treatment and serial imaging follow-up of PCLs in these patients.
However, there are limitations to this study. The retrospective nature, heterogeneous cohort, small sample size, and relatively short follow-up time may have limited the results. Future studies with more patients, longer follow-up times, and only a single type of organ transplantation are needed to determine the true effect of organ transplantation in patients with PCLs. PCLs are not uncommon in SOT recipients. In lesions without high-risk features, the development of features suggesting cancer risk is rare. These lesions can be managed conservatively, and their presence should not affect transplant eligibility or posttransplant management.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2022-00-01003-010).
Author Contributions
Conceptualization: SY, YDY, PJP. Data curation: SY, YJC, HSJ. Formal analysis: SY, YJC, YDY. Methodology: SY, YDY, PJP. Project administration: PJP, DSK. Visualization: HSJ. Writing–original draft: SY, YJC. Writing–review & editing: all authors.
REFERENCES
1. van Huijgevoort NC, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. 2019; Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 16:676–89. DOI: 10.1038/s41575-019-0195-x. PMID: 31527862.
2. Ip IK, Mortele KJ, Prevedello LM, Khorasani R. 2011; Focal cystic pancreatic lesions: assessing variation in radiologists' management recommendations. Radiology. 259:136–41. DOI: 10.1148/radiol.10100970. PMID: 21292867.
3. Girometti R, Intini S, Brondani G, Como G, Londero F, Bresadola F, et al. 2011; Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging. 36:196–205. DOI: 10.1007/s00261-010-9618-4. PMID: 20473669.
4. Chang YR, Park JK, Jang JY, Kwon W, Yoon JH, Kim SW. 2016; Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: large-scale, single-center cohort study. Medicine (Baltimore). 95:e5535. DOI: 10.1097/MD.0000000000005535. PMID: 28002329. PMCID: PMC5181813.
5. de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, et al. 2010; High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 8:806–11. DOI: 10.1016/j.cgh.2010.05.017. PMID: 20621679.
6. Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. 2012; International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 12:183–97. DOI: 10.1016/j.pan.2012.04.004. PMID: 22687371.
7. Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, et al. 2013; European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 45:703–11. DOI: 10.1016/j.dld.2013.01.010. PMID: 23415799.
8. Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. 2017; Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 17:738–53. DOI: 10.1016/j.pan.2017.07.007. PMID: 28735806.
9. European Study Group on Cystic Tumours of the Pancreas. 2018; European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 67:789–804. DOI: 10.1136/gutjnl-2018-316027. PMID: 29574408. PMCID: PMC5890653.
10. Al-Haddad M, Gill KR, Raimondo M, Woodward TA, Krishna M, Crook JE, et al. 2010; Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: a controlled study. Endoscopy. 42:127–32. DOI: 10.1055/s-0029-1215351. PMID: 19998218.
11. Thomas T, Bebb J, Mannath J, Ragunath K, Kaye PV, Aithal GP. 2010; EUS-guided pancreatic cyst brushing: a comparative study in a tertiary referral centre. JOP. 11:163–9.
12. Sendino O, Fernández-Esparrach G, Solé M, Colomo L, Pellisé M, Llach J, et al. 2010; Endoscopic ultrasonography-guided brushing increases cellular diagnosis of pancreatic cysts: a prospective study. Dig Liver Dis. 42:877–81. DOI: 10.1016/j.dld.2010.07.009. PMID: 20810331.
13. Basar O. 2018; Pancreatic cyst biopsy: improvement in diagnosis with micro forceps biopsy. Cancer Cytopathol. 126:227–8. DOI: 10.1002/cncy.21975. PMID: 29405615.
14. Stark A, Donahue TR, Reber HA, Hines OJ. 2016; Pancreatic cyst disease: a review. JAMA. 315:1882–93. DOI: 10.1001/jama.2016.4690. PMID: 27139061.
15. Vidhyarkorn S, Siripongsakun S, Yu J, Sayre J, Agopian VG, Durazo F, et al. 2017; Longterm follow-up of small pancreatic cystic lesions in liver transplant recipients. Liver Transpl. 23:324–9. DOI: 10.1002/lt.24680. PMID: 27875639.
16. Doycheva I, Amer S, Watt KD. 2016; De novo malignancies after transplantation: risk and surveillance strategies. Med Clin North Am. 100:551–67. DOI: 10.1016/j.mcna.2016.01.006. PMID: 27095645.
17. Buell JF, Brock GN. 2008; Risk of cancer in liver transplant recipients: a look into the mirror. Liver Transpl. 14:1561–3. DOI: 10.1002/lt.21634. PMID: 18975288.
18. Liu K, Joshi V, van Camp L, Yang QW, Baars JE, Strasser SI, et al. 2017; Prevalence and outcomes of pancreatic cystic neoplasms in liver transplant recipients. World J Gastroenterol. 23:8526–32. DOI: 10.3748/wjg.v23.i48.8526. PMID: 29358860. PMCID: PMC5752712.
19. Lennon AM, Victor D, Zaheer A, Ostovaneh MR, Jeh J, Law JK, et al. 2014; Liver transplant patients have a risk of progression similar to that of sporadic patients with branch duct intraductal papillary mucinous neoplasms. Liver Transpl. 20:1462–7. DOI: 10.1002/lt.23983. PMID: 25155689. PMCID: PMC4322915.
Fig. 1
Type of pancreatic cystic lesions. (A, B) Serous cystadenoma. (C) Mucinous cystic neoplasm (MCN) (H&E, ×200). (D) Intraductal papillary mucinous neoplasm (IPMN) (H&E, ×200).
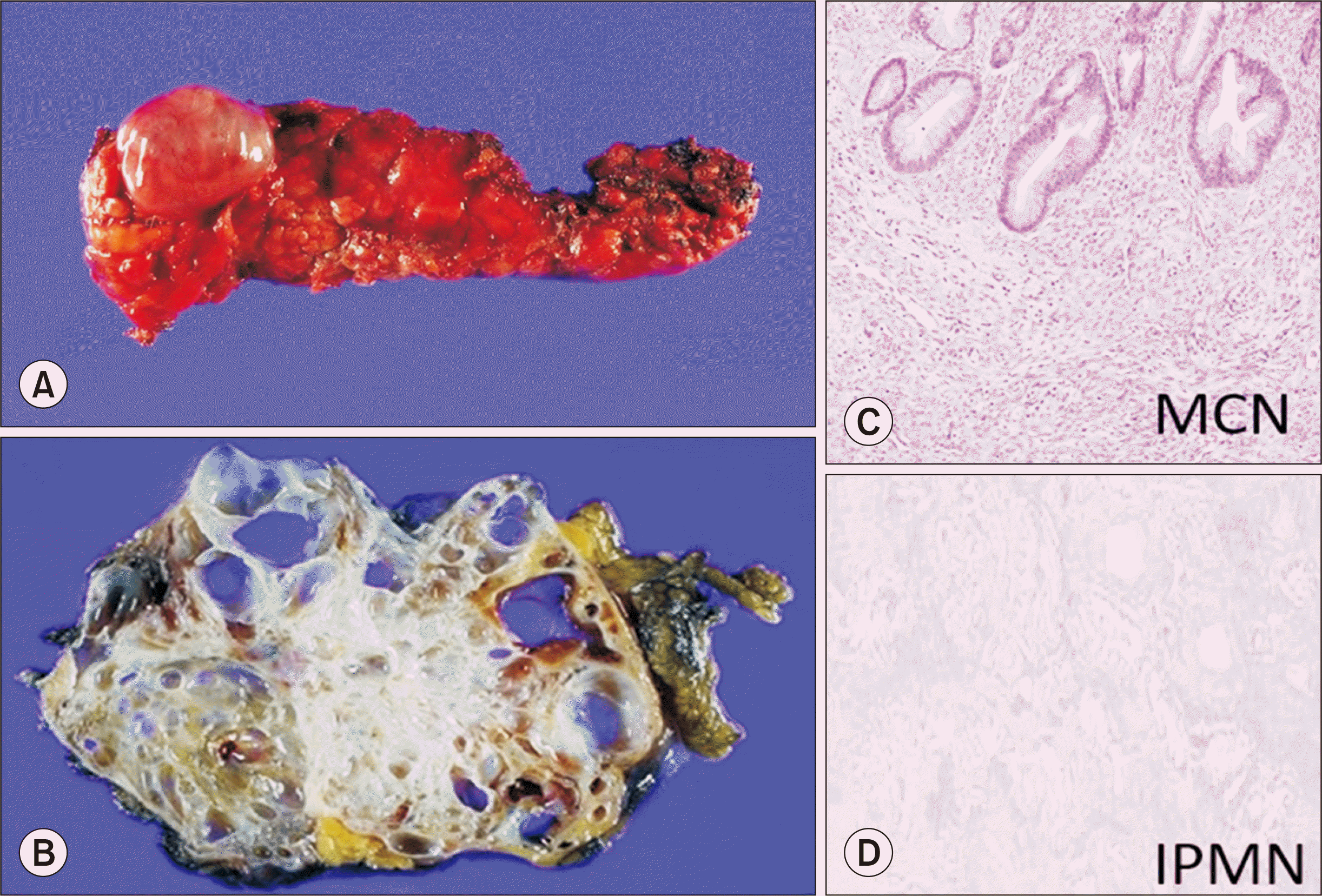
Table 1
Clinical characteristics of solid organ transplant recipients diagnosed with pancreatic cystic lesions
Table 2
Baseline characteristics and clinical outcomes of pancreatic cystic lesions
| Characteristic | SOT group (n=15) | Control group (n=60) | P-value |
|---|---|---|---|
| Type of PCL | 0.150 | ||
| SCA | 4 (26.7) | 24 (40.0) | |
| MCN | 1 (6.7) | 3 (5.0) | |
| IPMN | 6 (40.0) | 29 (48.3) | |
| Pseudocyst | 4 (26.7) | 4 (6.7) | |
| Number | 0.753 | ||
| Single | 13 (86.7) | 50 (83.3) | |
| Multiple | 2 (13.3) | 10 (16.7) | |
| Location | 0.057 | ||
| Head | 6 (40.0) | 20 (33.3) | |
| Body | 0 | 17 (28.3) | |
| Tail | 9 (60.0) | 23 (38.3) | |
| Cyst size (cm) | |||
| At diagnosis | 2.40±2.07 | 2.01±1.07 | 0.497 |
| At last imaging | 2.00±2.02 | 1.96±1.22 | 0.909 |
| Cyst change | 0.007 | ||
| Increase | 2 (13.3) | 18 (30.0) | |
| Decrease | 6 (40.0) | 5 (8.3) | |
| No change | 7 (46.7) | 37 (61.7) | |
| Worrisome feature | |||
| Cyst symptoma) | 0 | 0 | - |
| Mural nodule | 0 | 1 (1.7) | 1.000 |
| MPD dilatation | 2 (13.3) | 3 (5.0) | 0.260 |
| Follow-up (mo) | 56.40±36.71 | 41.73±28.80 | 0.100 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download