Abstract
Objective
This prospective, randomized, double-blinded trial aimed to evaluate the efficacy and safety of prophylactic ramosetron administration against postoperative nausea and vomiting (PONV) in patients undergoing microvascular decompression (MVD).
Methods
In this study, 100 patients undergoing MVD were randomly allocated to the control (normal saline, 2 mL) or ramosetron (ramosetron, 0.3 mg) groups at the end of surgery. The incidence and severity of PONV, need for rescue antiemetics, patient satisfaction score, duration of hospital stay, and the occurrence of adverse events were evaluated 48 hours post-surgery.
Results
Data obtained from 97 patients were included in the final analysis. The incidence of PONV was significantly lower in the ramosetron group than in the control group throughout the 48-hour postoperative period (29.2% vs. 51.0%, p=0.028). A similar trend was observed with regard to PONV severity (p=0.041). The need for rescue antiemetics, satisfaction score, duration of hospital stays, and the occurrence of adverse events did not significantly differ between the groups.
Postoperative nausea and vomiting (PONV) is one of the most common complications reported in patients following surgery and anesthesia, with an incidence of 30–80% [19]. PONV may decrease the quality of recovery and cause aspiration, intracranial hypertension, dehydration, acid-base disturbance, electrolyte imbalance, and neurological deterioration [5-7,18], which leads to a prolonged hospital stay and increased medical costs [12]. Therefore, PONV prophylaxis has become a critical issue for the enhancement of the quality of recovery and surgical outcomes.
The pathophysiology of PONV involves stimulation of various afferent pathways and emetic reflex activation [22]. Afferent pathways associated with PONV include the chemoreceptor trigger zone (CTZ), vagal mucosal pathway of the gastrointestinal system, neuronal pathway of the vestibular system, reflex afferent pathway of the cerebral cortex, or midbrain afferents [22]. Several pharmacological interventions, including anticholinergics, antihistamines, corticosteroids, dopamine receptor antagonists, and serotonin receptor antagonists, for treating and preventing PONV have been assessed [2,3].
Microvascular decompression (MVD), a treatment of choice for hemifacial spasm (HFS) or trigeminal neuralgia (TN) is associated with an increased risk of PONV, which occurs in >70% patients who undergo MVD [13]. HFS or TN is usually caused by the abnormal compression of a cranial nerve [16], and surgical procedures require a close approach to neural structures by the CTZ or vestibular systems to relieve irritated nerves [17]. Thus, surgical manipulation may predispose patients to PONV after MVD [21]. Several studies have been conducted to prevent PONV in patients undergoing MVD [23,25].
Ramosetron, a selective serotonin receptor antagonist, can effectively reduce the incidence of PONV after various types of surgeries, including neurosurgery [11,20]. However, whether ramosetron administration reduces the incidence of PONV after MVD remains unclear. We hypothesized that prophylactic use of ramosetron reduces the incidence of PONV after MVD. Therefore, this study aimed to determine the efficacy of prophylactic ramosetron administration in reducing the incidence and severity of PONV in patients undergoing MVD and to elucidate its safety.
The protocol of this prospective, double-blind, single-center, randomized controlled trial was approved by the Institutional Review Board of Seoul National University Bundang Hospital (protocol code B-2003/600-005 and date of approval April 7, 2020) and was registered within the UMIN Clinical Trials Registry (UMIN 000040178). Written informed consent was obtained from all eligible patients before surgery. The study was conducted according to the guidelines of the Declaration of Helsinki. Adult patients (aged >19 years) with an American Society of Anesthesiologists physical status I/II scheduled to undergo MVD under general anesthesia were enrolled in this trial. We excluded the following patients : those with a body mass index of <18.5 or >35.0 kg/m2; those with a history of craniotomy or anticancer chemotherapy, severe renal or hepatic dysfunction, or QTc prolongation; and those who used preoperative antiemetics within the 24-hour period before surgery, used opioids for >2 weeks, or were pregnant.
Patients were randomly allocated to one of two groups (control or ramosetron group) in a 1 : 1 ratio using computer-generated random sequences with a block size of 4 (random allocation software, version 2.0; Isfahan University of Medical Sciences, Isfahan, Iran). Each group was treated at the end of surgery, as follows : the control group, 2 mL saline; and the ramosetron group, 0.3 mg ramosetron (Nasea®, Daiichi-Sankyo Korea, Seoul, Korea). Identical 2-mL syringes containing either saline or 0.3-mg ramosetron were prepared and administered intravenously during dura closure. Random sequences were sealed in an opaque envelope and opened by an anesthesiologist who prepared the drug but was not further involved with the study. All patients, neurosurgeons, anesthesiologists, and outcome assessors were blinded to the group assignments.
A 0.02 mg/kg midazolam bolus was administered intravenously pre-surgery in the reception area. In the operating room, patients were monitored using electrocardiography, noninvasive blood pressure measurement, pulse oximetry, and bispectral index assessment (Medtronic, Minneapolis, MN, USA). After preoxygenation, general anesthesia was induced using propofol (4 mcg/mL) and remifentanil (3 ng/mL) with a target-controlled infusion pump (Fresenius Vial, Brezins, France). After loss of consciousness, 0.6 mg/kg rocuronium was administered as a neuromuscular blockade; subsequently, tracheal intubation was performed. Plain endotracheal tubes (Covidien, Mansfield, MA, USA) with inner diameters of 7.5 and 7.0 mm were used for male and female patients, respectively. Patients were ventilated with an inspired oxygen fraction of 0.5, a tidal volume of 6–8 mL/kg of ideal body weight, and a positive end-expiratory pressure of 5 cmH2O. The respiratory rate of each patient was controlled to maintain an end-tidal carbon dioxide level of 30–35 mmHg. Anesthesia was maintained at a bispectral index of 40–60 during surgery by adjusting the target concentration of either propofol (3.5–5.0 mcg/mL) or remifentanil (2.0–4.0 ng/mL). No additional rocuronium was administered for intraoperative neurophysiological monitoring. Hypotension, defined in this study as a mean blood pressure <60 mmHg, was treated with either 5 mg ephedrine or 20 mcg phenylephrine, as appropriate. At the end of surgery, 50 mcg/kg neostigmine and 10 mcg/kg glycopyrrolate were used to reverse effects of residual neuromuscular blockade, and a patient-controlled analgesia (PCA) device delivering 180 mg ketorolac (total volume, 100 mL) was connected intravenously. The PCA device (AutoMed 3200; Ace Medical, Seoul, Korea) was programmed to deliver a bolus dose of 2 mL, with a continuous 2 mL/h infusion rate, and a 15-minute lockout time. After patients could breathe continuously, they were extubated and transferred to the post-anesthesia care unit (PACU). Patients were discharged from the PACU when they had a modified Aldrete score ≥9 [24].
One neurosurgeon performed all MVDs, as described previously [9]. Briefly, with the patient in the supine position, the head was rotated approximately 20–30° away from the affected side without the use of head fixation. Brain retractors were not used during the surgical procedure. A 4–5-cm curvilinear skin incision, three quarters below the mastoid notch in patients with HFS and half above the mastoid notch in those with TN, was performed along the hairline. Following the identification of the digastric groove, a 2–2.5-cm craniectomy was performed below the digastric groove for HFS and above the digastric groove for TN. An incision of the dura mater was made along the inferoposterior margin of the sigmoid sinus. Subsequently, the arachnoid membrane/trabeculations along the involved cranial nerves were dissected carefully. The offending vessel(s) were decompressed from the cranial nerves after exploration of the entire intracranial portion of each of these involved nerves, including the root exit/entry zone and cisternal segment. A watertight dural closure was subsequently achieved. Finally, the deep and superficial muscles and the skin were approximated.
After surgery, a blinded outcome assessor evaluated patient outcomes at the following predefined time points : 1 hour, 24 hours, and 48 hours postoperatively. The primary outcome was the incidence of PONV throughout the 48-hour postoperative period. Based on a previous study [15], nausea was defined as an unpleasant feeling due to which the patient had the urge to vomit, and vomiting was defined as forceful expulsion of gastric contents from the mouth.
Secondary outcomes were PONV severity throughout the 48-hour postoperative period, rescue antiemetic requirements during the 48-hour postoperative period, PONV satisfaction score at postoperative day 2, and duration of hospital stay. The severity of PONV was evaluated using a 4-point scale, which was described in a previous study, as follows [23] : 0, no symptoms; 1, few mild symptoms but no requirement for treatment; 2, moderate symptoms that require treatment; and 3, severe, persistent symptoms after treatment. Intravenous metoclopramide (10 mg) was administered as a rescue antiemetic for patients who wanted treatment or experienced more than one episode of vomiting. On postoperative day 2, all patients were asked to provide satisfaction scores using an 11-point numerical rating scale, as follows : 0, very dissatisfied; 10, very satisfied. Adverse events such as constipation, or hiccups were also recorded.
According to a previous study [13], the incidence of PONV during the 48-hour postoperative period in patients undergoing MVD was 78%. A 30% decrease in the incidence of PONV was considered to be clinically significant, and 50 patients/group were deemed necessary to achieve type 1 error of 0.05 (false positive) and type 2 error of 0.2 (false negative) with a dropout rate of 20%.
Continuous variables are presented as means with standard deviations or medians with interquartile ranges and were compared using Student’s t-test or the Mann-Whitney U test based on whether the values were normally distributed. Whether data were normally distributed was determined using the Shapiro-Wilk test. Categorical variables are presented as numbers and percentages and were compared using chi-squared or Fisher’s exact test. All analyses were performed in an intention-to-treat manner. Univariate and multivariate logistic regression analyses were performed to adjust confounding variables. Variables included in the logistic regression model were female gender and non-smoker which are independent risk factors for development of PONV [1]. All statistical analyses were performed using Statistical Package for the Social Sciences, version 22, for Windows (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
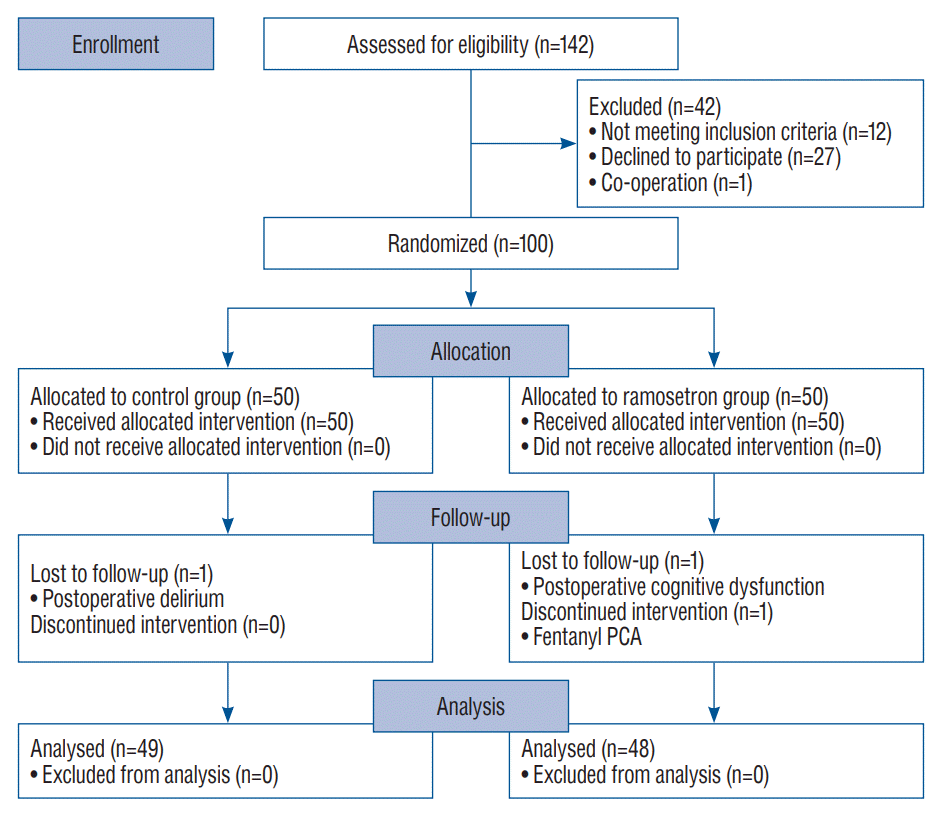
A total of 142 patients were considered for enrollment in the study from May 2020 to March 2021, and 42 were excluded (Fig. 1). Therefore, 100 patients were randomly placed in each study group, and data obtained from 97 were included in final analyses. The intervention was discontinued in one patient of the ramosetron group owing to a PCA (use of fentanyl instead of ketorolac), and two (one from each group) were lost to follow-up due to postoperative cognitive dysfunction or postoperative delirium (Fig. 1). Table 1 includes characteristics of patients, surgery, and anesthesia; no significant differences between groups were noted.
The incidence of PONV was significantly lower in the ramosetron group than in the control group (p=0.028) during the study period (Table 2). Effects of ramosetron on PONV reduction were significant on postoperative day 1 (1–24 hours) (p=0.040, Table 2). Additionally, the severity of PONV assessed using a 4-point scale was determined to be lower in the ramosetron group than in the control group (p=0.041, Table 2). The number of patients who received a rescue antiemetic drug was lower in the ramosetron group than in the control group, but the difference was not statistically significant (p=0.068, Table 2). Patient satisfaction scores in both groups were comparable (p=0.827, Table 2).
Duration of hospital stay did not significantly differ between the groups (p=0.731, Table 3). There was no significant difference between groups in the incidence of adverse events (Table 3). Six patients in the ramosetron group and two in the control group reported constipation (p=0.159). In the ramosetron group, two patients complained of hiccups (p=0.242). All symptoms were resolved by the day of discharge.
Although the control group has more female patients compared to the ramosetron group (57% vs. 50%), multivariate analysis revealed that the use of ramosetron could independently prevent the development of PONV (p=0.039, Table 4).
This is the first randomized clinical trial to assess the efficacy and safety of ramosetron, a 5HT3 antagonist, against PONV in patients undergoing MVD. Findings of the study suggest that prophylactic administration of ramosetron reduces the incidence and severity of PONV after MVD without causing any adverse events. However, the administration of ramosetron at the end of surgery did not affect rescue antiemetic use, or duration of hospital stay.
It is worth noting that ramosetron reduced both the incidence and severity of PONV after neurosurgery. In addition to causing postoperative pain, the occurrence of PONV is highly concerning after surgeries performed under anesthesia. Especially in patients undergoing neurosurgery, PONV may increase arterial and intracranial pressure, which can be confused with neurologic complications. The etiology of PONV is associated with patient traits, the anesthetic used, and surgical factors [8], although MVD has not been determined to be a surgical risk factor for PONV. However, many studies have suggested that MVD is a type of neurosurgery associated with a high risk of PONV. This is likely because the cranial nerve dissected during MVD is close to the vestibular nerve and nucleus of the brainstem [13,17].
The incidence of PONV after MVD has been reported to be approximately 70%, and in this study, its incidence rate in the control and ramosetron groups was approximately 51% and 29%, respectively. The relatively low incidence of PONV in the control group may be explained by the fact that the PCA regimen used in this study included non-steroidal anti-inflammatory drugs (ketorolac) instead of opioids. A previous univariate analysis that included PONV patients revealed that the use of a ketorolac-based PCA was not significantly associated with PONV [26]. Prophylactic antiemetic treatment with ramosetron decreased the incidence of PONV by >20% relative to that by control treatment. Few studies on the effects of other 5HT3 antagonists on PONV in patients who underwent MVD have been conducted thus far [10,23]. Thongrong et al. [23] conducted a prospective, randomized, controlled trial to assess the use of ondansetron with dexamethasone in patients who underwent MVD and found that intraoperative administration of the drug combination decreased the incidence of PONV 24 hours post-surgery by 15% versus control treatment (66.7% vs. 81.5%, respectively). Another retrospective observational study that assessed palonosetron use revealed that decreases in the incidence of PONV were higher when a combination of prophylactic palonosetron and sugammadex (reverses neuromuscular blockade) was administered versus control treatment (incidence, 19.3% vs. 37.2%, respectively) [10]. The incidence of PONV in this study was significantly lower than that previously reported in patients following MVD. Underreporting due to the retrospective nature of the study is possible. Lee et al. [13] studied the effect of transdermal scopolamine on PONV after MVD and found that treatment decreased the severity of PONV and rescue antiemetics use throughout the 48-hour postoperative period without affecting PONV incidence, which was high (approximately 70%).
Although PONV may prolong the duration of the hospital stay [12], the hospital stay duration did not significantly differ between the two groups assessed in this study. A possible explanation for this may be that only three patients (two in the control group and one in the ramosetron group) had severe and refractory PONV symptoms on postoperative day 2.
This study has some limitations. First, we did not record headaches, drowsiness, and dizziness, which are common adverse events related to ramosetron treatment [14,20]. However, it is difficult to distinguish between the adverse events associated with ramosetron and post-neurosurgical complications. Second, the severity of PONV was assessed using a 4-point scale, although an 11-point numerical rating scale might have provided more detailed information regarding the severity of PONV in the individuals included in this study. Third, the prophylactic effect of ramosetron seems to be significant up to postoperative day 1 (24 hours after surgery) although ramosetron is known to be effective up to 48 hours after surgery [4]. This waning effect of ramosetron after one day may explain the non-superiority in satisfaction scores of the ramosetron group. Additionally, it was difficult to validate the effect of ramosetron between the two groups after 24 hours due to low incidence of PONV during this period.
In conclusion, prophylactic administration of ramosetron reduced the incidence and severity of PONV in patients with MVD without causing serious adverse events. Thus, ramosetron administration may improve the quality of recovery in patients undergoing MVD. Further studies using a multimodal antiemetic approach are needed in patients with MVD to assess the enhancement in their quality of recovery.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author contributions
Conceptualization : JHR, JHH; Data curation : YKB, YTJ; Formal analysis : CHK, SYJ; Funding acquisition : JHR; Methodology : CHK, SYJ; Project administration : YTJ, JHR, JHH; Visualization : CHK; Writing - original draft : CHK, SYJ, YKB; Writing - review & editing : YTJ, JHR, JHH
References
1. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 91:693–700. 1999.
2. Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 107:i27–i40. 2011.
3. Cho YJ, Choi GJ, Ahn EJ, Kang H. Pharmacologic interventions for postoperative nausea and vomiting after thyroidectomy: a systematic review and network meta-analysis. PLoS One. 16:e0243865. 2021.
4. Choi YS, Shim JK, Yoon DH, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976). 33:E602–E606. 2008.
5. Fabling JM, Gan TJ, El-Moalem HE, Warner DS, Borel CO. A randomized, double-blinded comparison of ondansetron, droperidol, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. Anesth Analg. 91:358–361. 2000.
6. Fabling JM, Gan TJ, El-Moalem HE, Warner DS, Borel CO. A randomized, double-blind comparison of ondansetron versus placebo for prevention of nausea and vomiting after infratentorial craniotomy. J Neurosurg Anesthesiol. 14:102–107. 2002.
7. Fabling JM, Gan TJ, Guy J, Borel CO, El-Moalem HE, Warner DS. Postoperative nausea and vomiting. a retrospective analysis in patients undergoing elective craniotomy. J Neurosurg Anesthesiol. 9:308–312. 1997.
8. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 131:411–448. 2020.
9. Go KO, Hwang K, Han JH. Surgical nuances to reduce and manage cerebrospinal fluid leaks after microvascular decompression. J Clin Med. 9:902. 2020.
10. Kang HY, Park SW, Lee S, Jeon JM, Oh ID, Choi JH. Effect of prophylactic palonosetron and sugammadex on postoperative nausea and vomiting in patients undergoing microvascular decompression under propofolmaintained anesthesia: a retrospective observational study. Medicine (Baltimore). 97:e13237. 2018.
11. Kim WO, Koo BN, Kim YK, Kil HK. Ramosetron for the prevention of postoperative nausea and vomiting (PONV): a meta-analysis. Korean J Anesthesiol. 61:405–412. 2011.
12. Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 59:213–243. 2000.
13. Lee HH, Kim HM, Lee JE, Jeon YT, Park S, Hwang K, et al. The effect of a transdermal scopolamine patch on postoperative nausea and vomiting after retromastoid craniectomy with microvascular decompression: a preliminary single center, double-blind, randomized controlled trial. J Clin Med. 9:156. 2020.
14. Lee SY, Lee JY, Park SY, Kim JH, Cho OG, Kim JS, et al. Prophylactic antiemetic efficacy of granisetron or ramosetron in patients undergoing thyroidectomy. Asian J Surg. 25:309–314. 2002.
15. Madenoglu H, Yildiz K, Dogru K, Kurtsoy A, Güler G, Boyaci A. Randomized, double-blinded comparison of tropisetron and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. J Neurosurg Anesthesiol. 15:82–86. 2003.
16. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 90:1–8. 1999.
17. Meng L, Quinlan JJ. Assessing risk factors for postoperative nausea and vomiting: a retrospective study in patients undergoing retromastoid craniectomy with microvascular decompression of cranial nerves. J Neurosurg Anesthesiol. 18:235–239. 2006.
18. Neufeld SM, Newburn-Cook CV. The efficacy of 5-HT3 receptor antagonists for the prevention of postoperative nausea and vomiting after craniotomy: a meta-analysis. J Neurosurg Anesthesiol. 19:10–17. 2007.
19. Pierre S, Whelan R. Nausea and vomiting after surgery. CEACCP. 13:28–32. 2013.
20. Ryu JH, Lee JE, Lim YJ, Hong DM, Park HP, Han JI, et al. A prospective, randomized, double-blind, and multicenter trial of prophylactic effects of ramosetronon postoperative nausea and vomiting (PONV) after craniotomy: comparison with ondansetron. BMC Anesthesiol. 14:63. 2014.
21. Sato K, Sai S, Adachi T. Is microvascular decompression surgery a high risk for postoperative nausea and vomiting in patients undergoing craniotomy? J Anesth. 27:725–730. 2013.
22. Shaikh SI, Nagarekha D, Hegade G, Marutheesh M. Postoperative nausea and vomiting: a simple yet complex problem. Anesth Essays Res. 10:388–396. 2016.
23. Thongrong C, Chullabodhi P, Kasemsiri P, Kitkhuandee A, Plailaharn N, Sabangban L, et al. Effects of intraoperative dexamethasone and ondansetron on postoperative nausea and vomiting in microvascular decompression surgery: a randomized controlled study. Anesthesiol Res Pract. 2018:6297362. 2018.
24. Trevisani L, Cifalà V, Gilli G, Matarese V, Zelante A, Sartori S. Postanaesthetic discharge scoring system to assess patient recovery and discharge after colonoscopy. World J Gastrointest Endosc. 5:502–507. 2013.
25. Xu G, Zhao J, Liu Z, Liu G, Liu L, Ren C, et al. Dexmedetomidine combined with butorphanol or sufentanil for the prevention of postoperative nausea and vomiting in patients undergoing microvascular decompression: a randomized controlled trial. Front Med (Lausanne). 7:583031. 2020.
26. Yi MS, Kang H, Kim MK, Choi GJ, Park YH, Baek CW, et al. Relationship between the incidence and risk factors of postoperative nausea and vomiting in patients with intravenous patient-controlled analgesia. Asian J Surg. 41:301–306. 2018.
Table 1.
Demographic characteristics of patients, surgery, and anesthesia
Table 2.
Postoperative nausea and vomiting
| Control group (n=49) | Ramosetron group (n=48) | p-value | |
|---|---|---|---|
| Incidence of PONV | 25 (51.0) | 14 (29.2) | 0.028* |
| 0–1 hour | 11 (22.4) | 5 (10.4) | 0.110 |
| 1–24 hours | 22 (44.9) | 12 (25.0) | 0.040* |
| 24–48 hours | 10 (20.4) | 8 (16.7) | 0.636 |
| Severity of PONV | 1 (0–2) | 0 (0–1) | 0.041† |
| Rescue antiemetics‡ | |||
| Number of requirements | 0 (0–1) | 0 (0–0) | 0.073 |
| Number of patients | 16 (32.7) | 8 (16.7) | 0.068 |
| Satisfaction score | 10 (8–10) | 9.5 (8–10) | 0.827 |
Table 3.
Duration of hospital stay, and adverse events
| Control group (n=49) | Ramosetron group (n=48) | p-value | |
|---|---|---|---|
| Duration of hospital stay (days) | 3 (3–4) | 3 (3–4) | 0.731 |
| Adverse events | |||
| Constipation | 2 (4.1) | 6 (12.5) | 0.159 |
| Hiccups | 0 (0.0) | 2 (4.2) | 0.242 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download