Abstract
Objective
Chiari III malformations are extremely rare hindbrain malformations that are associated with a high early mortality rate, or severe neurologic deficits in the survivors. The treatment is early operative closure and cerebrospinal fluid diversion (CSF) shunting.
Methods
We operated on 15 patients by repair and excision between July 2014 till June 2020 and retrospective data collection was done. Only one patient doesn’t need ventriculoperitoneal (VP) shunt and the other 14 patients need a VP shunt. We described stepwise dissection and untethering of the cerebellum from the bony edge to regrowth and herniation of cerebellum again into this potential space and simple dural closure or repair with graft was done.
Results
We started with VP in eight patients (53%) and the other seven patients (46.7%) started with excision and then six patients need VP shunt later on because four patients developed CSF leak and two patients developed increased high intracranial tension. Only four patients (26.7%) needed a blood transfusion.
Chiari malformations (CMs) are a group of deformities in the hindbrain that are linked to spinal abnormalities and hydrocephalus. they were first described by Hans Chiari [20]. Chiari type III is the most uncommon of the CMs, and it is characterized anatomically by the presence of a high cervical or occipital encephalocele, as well as several of the abnormalities seen in type II, such as a shallow posterior cranial fossa, caudal migration of cerebellar tonsils, medullary kinking, beaked tectum, and hydrocephalus [3]. Due to its rarity, little is known about the natural history, treatment, and postoperative course of this specific deformity, as data from the literature is limited and focuses mostly on structural and neuroradiological characteristics rather than clinical aspects [6,11]. The cornerstone of the treatment is the closure of the dura and cerebrospinal fluid diversion (CSF) for the hydrocephalus before or after excision [10,13,19]. Unfortunately, a very poor prognosis was reported [3,17,19]. Here we report the presentation and outcome of 15 cases operated upon in our high-flow institute serving more than 25 million people.
This study was approved by the Ethical Committee of the neurosurgery department with Institutional Review Board of Cairo University Hospital approval in November 2020. Our hospital is located in downtown Cairo and serves about 20-25 million people in this region. We also receive referrals from other parts of the country, with an estimated population of 100 million people, this is large because our center is the only well-known center for pediatric neurosurgery in Egypt. Between July 2014 and June 2020 retrospective analysis of patients’ registration data was done revealing 15 patients, all demographics were collected, pre and post-operative radiographs (computed tomography [CT] and magnetic resonance imaging [MRI] brain and cervical spine) were revised, and followup data were analyzed for the outcome and survival analysis. We excluded patients with cardiac anomalies, respiratory distress, and major sinus venous anomaly. We also excluded swellings that ruptured before surgery.
Among the 15 patients recorded, nine were females and six were males, the mean age was 8.47±5.74 months, the average head circumference was 46.53±2.45 cm, the average width of the anterior fontanelle was 3.8±1.08 cm, the calculated cyst size mean was 27.67±13.85 cm3. Five patients had developmental abnormalities, two with hypotonia, two had limb weakness, one had seizures, and one had spasticity in the four limbs. Four patients had associated anomalies with the cyst, one had corpus callosum agenesis, one had left temporal arachnoid cyst, one had polydactyly, and one had polydactyly with polycystic kidney syndrome. After reviewing the operative records, eight patients had VP shunt preoperative, those patients had confirmed symptoms and signs of increased intracranial pressure, and MRI brain showed ventriculomegaly, five patients had their shunt installed in the same setting, and the other three patients operated on few days before cyst excision. Six cases needed shunts after removal of the cyst, this was legitimized by the CSF leak from the wound, or ventriculomegaly on the follow-up CT brain, only one patient did not need CSF diversion.
A 1-month-old female child presented to us with a swelling at the back of the neck since birth. the child was good crying and spontaneous movements in four limbs. There was no significant increase in the size of swelling since birth. There was a history of medicinal drug intake by the mother during the antenatal period, but the details were not clear. On local examination, head circumference was 48 cm. the swelling was 42 cm sized, soft, fluctuant, partially compressible, transilluminant, nonpulsatile and nontender swelling in the occipital region with a positive cough impulse. The overlying skin was normal with no discharge from swelling (Figs. 1 and 2).
The operations were carried out after the risks and advantages of the surgery were explained and informed consent was obtained. Suboccipital craniectomy was performed on all patients following the linear paramedian incision along the encephalocele during surgery. Furthermore, individuals with posterior arch anomalies had C1-C2 laminectomy. A plane was created between the overlying skin and the encephalocele sac. The dura was opened with a linear incision after prepping the encephalocele’s neck. Sharp dissection was used to remove macroscopically recognized neural structures from the encephalocele’s interior membrane. While attempting to preserve as much neural tissue and vascular structures as possible, the sac and aberrant neural tissues within the encephaloceles were removed. The dura was then sutured watertight without constricting the encephalocele’s neck. An autologous big graft was employed in some cases. There was no usage of fibrin glue or any other type of tissue adhesive. The skin was then closed in layers. So our recommendation for stepwise dissection is summarized in 1) the cerebellum in the sac is amputated at the bony edge because it is nonfunctioning as it becomes contused avascular tissue as it is pushed by pulsation into the sac across the bony edge. Removed cerebellar tissue help for dural repair along the bony edge, the herniated cerebellum is dysplastic. 2) Closure the track completely to minimize CSF leak. 3) Untethering of the cerebellum from the bony edge to avoid the growth and recurrence of the cerebellum again because this old space that the cerebellum used to grow. And 4) any collection or leak is common, so proceed for shunt due to small posterior fossa and better to do repair with graft.
Most authors agreed that the Chiari III malformation is presented with cervical and/or occipital encephalocele with inferior and posterior displacement of cerebellar tissue into the encephalocele [2,5,6,15]. Young et al. [21] reported the difference between cervical myelomeningocele and occipital encephalocele as a distinct entity that was previously misdiagnosed as CM. The presentations are variable, and they ranged from asymptomatic newborns to a child presented with seizures, severe neurological deficit, or developmental delay4). Hydrocephalus is the most commonly associated anomaly [1,15].
Surgical repair is the main management to prevent rupture and infection [4,7,19]. The primary goal is the closure of the defect and the excision of neural tissue should be consevative [19] and avoidance of brain stem compression [11].
Chiari type III has a poor prognosis as it is associated with early death or severe disability in survivors [4]. We present our series of 15 cases of Chiari type III malformation, to our knowledge, this is the most reported number of cases till now, as the eight cases reported by Işik et al. [8] have the largest number of cases. In their case series, they evaluated the outcome of surgical repair and discussed the literature of Chiari III presentations and management [8]. Our series included nine females and six males, with a mean age of 8.47 months, we suggest that this high mean of age is mainly due to delayed referral of cases from peripheral parts of the country. Işik et al. [8] reported six males and two females, with a mean age of 148 days (ranging from 2 days to 2 years).
Other publications were only case reports, Sirikci et al. [18] reported an unusual presentation in an 11 years old girl, Jaggi and Premsagar [10] presented a 1-month-old girl with a neck swelling. To our knowledge, this is one of the largest numbers of cases of Chiari type III presented in a single study. We have found that most of the cases have associated anomalies, especially those related to neural tube development, this comes in accordance with Sirikci et al. [18] who found that most cases were associated with delayed milestones or motor dysfunction. Despite the fact that we were unable to locate any statistical data, we assume that a large number of newborns with an increase in CM III is caused by neural tube abnormalities. Our institute performs about 150–200 cases of CMs per year, this makes the incidence of Chiari type II malformations range from 1.8–2.5%, which falls in the international range from 0.6–4% [4,19].
Breathing and swallowing difficulties, as well as long tract abnormalities, are common symptoms of Chiari type III. Other symptoms include seizures, developmental delay, and brain stem nuclei disruption [9].
The timing of CSF diversion in patients with type III CM has been controversial for a long time, little data has been gathered but most authors combine CSF diversion with the operative closure of the defect in the same setting [19], and some surgeons give a chance to test the operative closure especially when there are no obvious symptoms of hydrocephalus, they usually postpone the shunt insertion to avoid lengthening the surgical time in an otherwise very young child [10].
We have performed CSF diversion on eight patients prior to surgical closure, and six patients required post-operative shunting, while only one case did not undergo shunt insertion. Despite the fact that we know very little about the natural history and outcome of CM III, we know that patients with this anomaly have a worse prognosis than patients with CM I or CM II and that it is generally associated with a high early mortality rate and/or severe neurological manifestations in patients who survive, as shown by previously reported cases [12,14,16].
Most complications arise from the irreversible damage to the cerebellar tissue, others are related to the surgical procedure itself, they vary from neurological deficits and delayed milestones to skin gapping and meningitis. We did not report any case of meningitis even in cases of CSF leak, this may be correlated to the usual use of prophylactic antibiotics (ceftriaxone and amikacin) 1 day preoperative and 5 days later. Also, we have recorded two cases of nystagmus, and one case of generalized hypotonia.
In many cases of Chiari type III malformation, and despite the associated anomalies and complications, surgical intervention may be the only salvage procedure that can help save the life of the affected child.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
1. Aleksic S, Budzilovich G, Greco MA, Reuben R, Feigin I, Pearson J, et al. Cerebellocele and associated central nervous system anomalies in the meckel syndrome. Childs Brain. 11:99–111. 1984.
2. Aribal ME, Gürcan F, Aslan B. Chiari III malformation: MRI. Neuroradiology. 38:S184–S186. 1996.
3. Caldarelli M, Rea G, Cincu R, Di Rocco C. Chiari type III malformation. Childs Nervous System. 18:207–210. 2002.
4. Cama A, Tortori-Donati P, Piatelli GL, Fondelli MP, Andreussi L. Chiari complex in children--neuroradiological diagnosis, neurosurgical treatment and proposal of a new classification (312 cases). Eur J Pediatr Surg 5 Suppl. 1:35–38. 1995.
5. Castillo M, Quencer RM, Dominguez R. Chiari III malformation: imaging features. AJNR Am J Neuroradiol. 13:107–113. 1992.
6. Chiari H. Ueber veränderungen des kleinhirns infolge von hydrocephalie des grosshirns. Dtsch Med Wochenschr. 17:1172–1175. 1891.
7. Häberle J, Hülskamp G, Harms E, Krasemann T. Cervical encephalocele in a newborn--Chiari III malformation. case report and review of the literature. Childs Nerv Syst. 17:373–375. 2001.
8. Işik N, Elmaci I, Silav G, Celik M, Kalelioğlu M. Chiari malformation type III and results of surgery: a clinical study: report of eight surgically treated cases and review of the literature. Pediatr Neurosurg. 45:19–28. 2009.
9. Ivashchuk G, Loukas M, Blount JP, Tubbs RS, Oakes WJ. Chiari III malformation: a comprehensive review of this enigmatic anomaly. Childs Nerv Syst. 31:2035–2040. 2015.
10. Jaggi RS, Premsagar IC. Chiari malformation type III treated with primary closure. Pediatr Neurosurg. 43:424–427. 2007.
11. Kannegieter LS, Dietrich RB, Pais MJ, Goldenberg TM. Pediatric case of the day. Chiari III malformation. Radiographics. 14:452–454. 1994.
12. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr (Phila). 34:650–654. 1995.
13. Lee R, Tai KS, Cheng PW, Lui WM, Chan FL. Chiari III malformation: antenatal MRI diagnosis. Clin Radiol. 57:759–761. 2002.
14. MacLellan DL, Bauer SB. Neuropathic dysfunction of the lower urinary tract. Campbell-Walsh Urology. Elsevier, 2012, pp3431–3456. e8.
15. Maroun FB. Syringomyelia and the chiari malformations. Can J Neurol Sci. 25:175. 1998.
16. Menezes AH, Smoker WRK, Dyste GN. Syringomyelia, chiarimalformations, and hydromyelia. In : Youmans JR, editor. Neuro-logical Surgery. Philadelphia: W. B. Saunders Co;1990. p. 1421–1459.
17. Pittman HW. The Chiari crisis. BNI Quart. 6:10–16. 1990.
18. Sirikci A, Bayazit YA, Bayram M. The Chiari III malformation: an unusual and asymptomatic variant in an 11-year old child. Eur J Radiol. 39:147–150. 2001.
19. Snyder WE Jr, Luerssen TG, Boaz JC, Kalsbeck JE. Chiari III malformation treated with CSF diversion and delayed surgical closure. Pediatr Neurosurg. 29:117–120. 1998.
20. Tubb RS, Pugh A, Oakes WJ. Chiari Malformations, 6th ed. Elsevier Inc;1918.
21. Young RM, Shafa JS, Myseros JS. The Chiari 3 malformation and a systemic review of the l-terature. Pediatr Neurosurg. 50:235–242. 2015.
Fig. 1.
A : computed tomography brain showing bony defect in posterior fossa wall with herniated cerebellum in sac. b : Magnetic resonance imaging (MRI) showing the sac. Arrows indicate herniated cerebellum. c : Axial MRI showing the encephalocele. Arrow indicates herniated cerebellum.
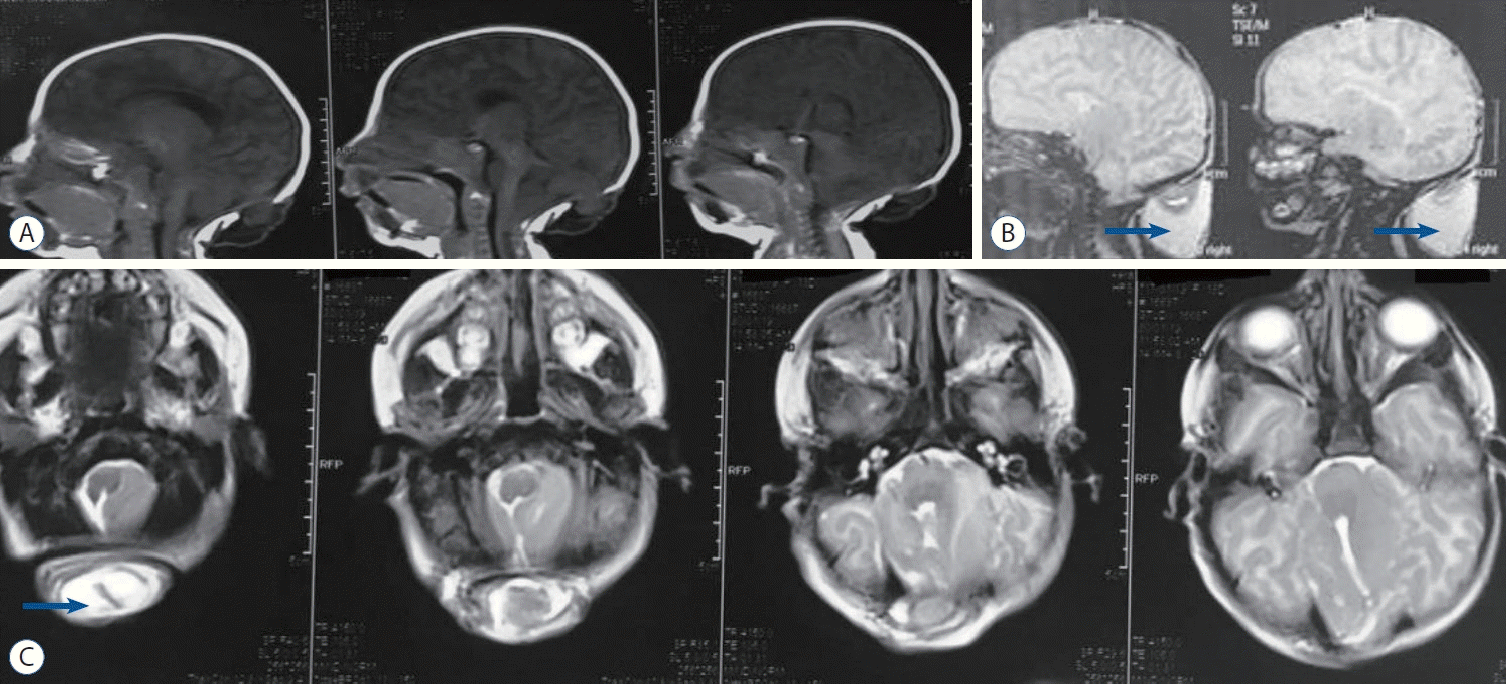
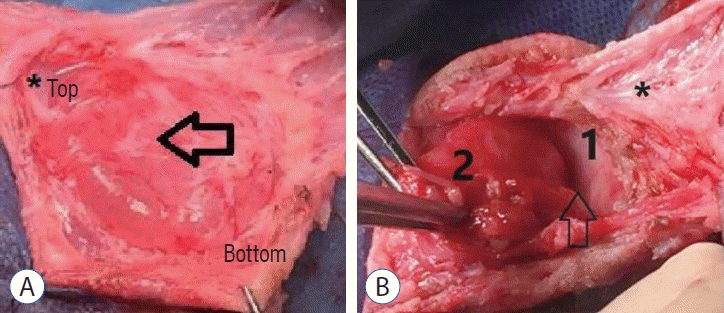
Fig. 2.
Intraoperative photo showing the stepwise dissection in (A) and (b) showing reflected dura 1 and excision of contused cerebellum in 2. A : Arrow refer to the capsule of the sac asterisk showing the plane of dissection. b : 1 refer to dura and 2 refer to cerebellar tissue, arrow; refer to bony defect and origin of encephalocele, asterisk refer to dissected capsule.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download