Abstract
Background/Aims
Digital single-operator cholangioscopy (DSOC)-guided mapping biopsy (DMB) and tube-assisted mapping biopsy (TMB) are two techniques used for preoperative evaluation of biliary tract cancer (BTC). However, data regarding the diagnostic performance of these techniques are limited.
Methods
We retrospectively examined consecutive patients with BTC who underwent either technique at our institution between 2018 and 2020. We evaluated the technical success rate, adequate tissue acquisition rate, and diagnostic performance of these techniques for the evaluation of lateral spread of BTC.
Results
A total of 54 patients were included in the study. The technical success rate of reaching the target sites was 95% for DMB and 100% for TMB. The adequate tissue acquisition rate was 61% for DMB and 69% for TMB. The adequate tissue acquisition rate was low, especially for target sites beyond the secondary biliary radicles. The sensitivity of DMB alone was 39%, which improved to 65% when combined with visual impression. Experts demonstrated a higher negative predictive value and diagnostic accuracy with respect to both DSOC visual impression and DMB for the evaluation of lateral spread of BTC compared to trainees.
Biliary tract cancer (BTC) often extends longitudinally along the biliary tract, precluding curative resection.1 Precise preoperative evaluation of lateral spread of BTC is required to determine surgical resectability and appropriate resection lines. Conventional imaging modalities such as multidetector-row computed tomography, magnetic resonance imaging, intraductal ultrasonography, and endoscopic ultrasound are useful in predicting the longitudinal spread of BTC2-6; however, it does not exclude the need for histological confirmation. Endoscopic transpapillary mapping biopsy under fluoroscopy is the most commonly used technique for tissue acquisition and is useful in evaluating intraductal tumor spread of BTC.7-9 However, the limited maneuverability of biopsy forceps often makes it difficult to advance them beyond the biliary stricture, particularly beyond secondary biliary radicles. Peroral cholangioscopy (POCS)-guided biopsy is a technique used for the diagnosis of indeterminate biliary strictures.10-13 Although POCS-guided mapping biopsy can be useful in preoperative evaluation of BTC,14,15 several issues remain unresolved. First, POCS requires a high level of expertise, which could affect its diagnostic accuracy.16 Second, only small caliber forceps with a 1-mm diameter cup can pass through the working channel of the cholangioscope. The small size of the obtained specimen may limit the ability to discriminate between benign and malignant lesions. Third, the cost-effectiveness of this expensive technique has not been fully evaluated.
Tube-assisted biopsy is another new technique that has recently been reported to be useful for the diagnosis of indeterminate biliary strictures.17 Despite its inability to perform targeted biopsies, it has several advantages over POSC-guided biopsy, including easier insertion beyond secondary biliary radicles, the ability to use larger caliber forceps, and much lower cost. While its utility for preoperative evaluation of BTC has recently been reported,18 direct comparisons with POCS-guided mapping biopsy have not been conducted to date. Therefore, we conducted this study to compare the utility of these two techniques in the preoperative evaluation of BTC.
We retrospectively examined consecutive patients who underwent digital single-operator cholangioscopy (DSOC)-guided mapping biopsy (DMB) or tube-assisted mapping biopsy (TMB) for preoperative evaluation of BTC at our institution between April 2018 and October 2020.
Endoscopic retrograde cholangiopancreatography (ERCP) was performed using a therapeutic duodenoscope (JF260, TJF260; Olympus Medical Systems, Tokyo, Japan) under moderate sedation with intravenous pethidine and midazolam. We used a tapered catheter (MTW ERCP catheter; MTW Endoskopie, Wesel, Germany) and a 0.025-inch guidewire (Visiglide2, Olympus Medical Systems; or Endoselector, Boston Scientific, Marlborough, MA, USA) for selective biliary cannulation in most cases.
For DMB procedures, endoscopic sphincterotomy using a sphincterotome (CleverCut3V; Olympus Medical Systems) was performed or had been performed previously in all patients before performing DSOC. The cholangioscope (SpyGlass DS Direct Visualization System or SpyGlass DS II Direct Visualization System; Boston Scientific) was inserted through the working channel of a therapeutic duodenoscope (TJF260) and advanced into the biliary tree over the guidewire. After the cholangioscope passed through the biliary stricture, the guidewire was withdrawn. Suction and irrigation were performed to obtain a clear view of the bile duct. First, the extent of lateral spread of the tumor was assessed by the treating endoscopists based on visualized cholangioscopic findings. Next, DMB was conducted under direct visualization using micro biopsy forceps (SpyBite or SpyBite Max; Boston Scientific) with a 1-mm diameter cup.
Endoscopic sphincterotomy was performed at the discretion of the treating endoscopists for the TMB procedures. We used the 7 Fr-delivery system of a plastic stent (Through & Pass; Gadelius Medical, Tokyo, Japan), which consists of an inner catheter and a pusher catheter, as a sheath. First, we advanced the delivery system slightly beyond the target site over a guidewire. Second, we removed the inner catheter and guidewire so that the pusher catheter remained in the bile duct slightly beyond the target site. A radio-opaque marker is located at the tip of the pusher catheter, which makes it easy to adjust the position of the catheter towards the target site. Third, a conventional biopsy forceps (Radial Jaw 4; Boston Scientific) with a 1.8-mm diameter cup was advanced through the pusher catheter to the target site under fluoroscopic guidance (Fig. 1). Cholangiograms could be obtained through the catheter between biopsies when needed.
Both procedures were performed by experts (≥50 cases of POCS experience) or trainees (<50 cases of POCS experience) under the guidance of experts. The mapping biopsy sites were determined following a discussion with the surgeons and included the following sites: the B2/3 confluence, B4 confluence, left hepatic duct, B5/B8 confluence, B6/B7 confluence, anterior segmental duct, posterior segmental duct, confluence of anterior and posterior segmental ducts, right hepatic duct, confluence of right and left hepatic ducts, upper bile duct, cystic duct confluence, and lower bile duct.
The diagnosis of BTC was based on pathological findings of bile aspiration cytology, brush cytology, or transpapillary forceps biopsy. Technical success of the procedure was defined as the successful biopsy of all the intended target sites. The extent of lateral tumor spread was determined based on surgical specimens. The severity of adverse events was graded according to the American Society of Gastrointestinal Endoscopy lexicon guidelines.19
The primary outcome was an adequate tissue acquisition rate for each target site. Tissue was considered adequate if it contained biliary epithelium; a specimen that contained only fibrous or connective tissue was considered inadequate. We evaluated the adequate tissue acquisition rates of each biopsy and the biopsy sites for both DMB and TMB using the entire cohort.
The secondary outcome was the diagnostic accuracy of the lateral spread of BTC, as confirmed by surgical specimens. Since only six patients underwent surgical resection for TMB, we focused on the 27 patients who underwent surgical resection for DMB. We also evaluated the impact of endoscopist expertise on the diagnostic performance of DSOC (visual impression, targeted biopsy, and visual impression plus targeted biopsy). The endoscopists’ interpretations of the DSOC findings were documented for each mapping biopsy site based on the endoscopic examination report written by the treating endoscopist. The endoscopists judged the sites as malignant when irregular tortuous vessels, irregular papillary or granular lesions, or nodular elevated lesions extended continuously from the main lesion. The pathological findings of biopsy specimens were defined as follows: adenocarcinoma or suspected adenocarcinoma was considered malignant, while atypical cells were considered benign. When either of the results of visual impression or targeted biopsy were malignant, the results of visual impression plus targeted biopsy were considered malignant.
Continuous variables were presented as medians (range) and were compared using the Mann-Whitney U-test. Categorical variables were described as absolute numbers (proportions) and were analyzed using the chi-square test or Fisher exact test, as appropriate. Statistical tests were two-sided, and a p<0.05 was considered statistically significant. Statistical analysis was performed using EZR ver. 1.40.20
This study was approved by the ethics committee of Cancer Institute Hospital of Japanese Foundation for Cancer Research (IRB No: 2021-1078) and was performed in accordance with the Declaration of Helsinki. Written informed consent for this study was waived by the ethics committee because of the retrospective design. A notification of this study was publicized on our hospital website, allowing patients to opt out of the study.
A total of 54 consecutive patients with BTC underwent preoperative DMB or TMB at our institution between April 2018 and October 2020 (Fig. 2). Since the cholangioscope could not be advanced through the biliary stricture in two patients, the technical success rate of reaching the target sites was 95% (39/41) for DMB and 100% (13/13) for TMB (p>0.999). Targeted biopsy was successful in all patients, except for two patients who failed to reach the target site. Thus, the 52 patients who successfully performed mapping biopsy comprised cohort 1. The patient and procedural characteristics of the two techniques are illustrated in Table 1. Although DMB had a higher proportion of men and a higher rate of prior biliary stenting compared to TMB, other characteristics including location of the stricture, total bilirubin level, papillary intervention, operator experience, and procedure time were similar between the two groups. The total number of mapping biopsy sites was 148 in the DMB and 45 in the TMB. The mean number of biopsies per site was 1.9 (range, 1–4) for DMB and 1.7 (range, 1–3) for TMB (p=0.211). Adverse events were also similar between the two groups; cholangitis occurred in four patients (10%) that received DMB and two patients (15%) that received TMB. No severe adverse events were observed in the present study.
The adequate tissue acquisition rates per biopsy of the two techniques are shown in Table 2. There were no significant differences in the adequate tissue acquisition rate per biopsy between the two groups. The overall adequate tissue acquisition rate per biopsy for nonstenotic bile duct sites was similar between the two groups: 61% for DMB and 69% for TMB (p=0.233). Adequate tissue acquisition rates per biopsy for target sites beyond secondary biliary radicles were low in both groups: B2/3 confluence (50% vs. 75%), B5/B8 confluence (52% vs. 86%), B6/B7 confluence (0% vs. 33%), anterior segmental duct (29% vs. 0%), and posterior segmental duct (33% vs. 0%). The overall adequate tissue acquisition rate per biopsy site was also similar between the two groups (74% vs. 82%, p=0.322).
Of the 52 patients who underwent DMB or TMB, 33 underwent surgical resection and comprised cohort 2. The reasons for inoperability were as follows: insufficient remnant liver volume and impaired liver function in seven patients, locally advanced disease after preoperative staging in six patients, unresectable disease confirmed by exploratory laparotomy in five patients (liver metastasis in two patients, peritoneal dissemination in two patients, and locally advanced disease in one patient), and lung metastasis just before surgery in one patient. Six patients were diagnosed as locally advanced disease by the results of ERCP (three patients each in the DMB group and TMB group). The former three patients were diagnosed as locally advanced disease by the results of DSOC (visual impression plus targeted biopsy). The latter three patients were diagnosed as locally advanced disease by the results of tube-assisted mapping biopsy and intraductal ultrasonography. In the TMB group, two patients showed extensive tumor spread to the hepatic side of the bile duct (confluence of anterior and posterior segmental ducts) and one patient showed extensive tumor invasion to the right hepatic artery, detected by intraductal ultrasonography. Table 3 shows the pathological characteristics of the patients who underwent surgical resection. Pathological diagnoses included hilar cholangiocarcinoma in 16 patients, distal cholangiocarcinoma in 12 patients, cystic duct carcinoma in three patients, and intrahepatic cholangiocarcinoma in two patients. The macroscopic type was flat in 17 patients, nodular in 12 patients, papillary in two patients, mass-forming in one patient, and unknown in one patient. The total number of evaluable biopsy sites was 106 and 16 sites in the DMB and TMB groups, respectively.
Of the 106 evaluable biopsy sites in the DMB group, 12 were true positives, 73 were true negatives, two were false positives, and 19 were false negatives. The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of DMB were 39%, 97%, 86%, 79%, and 80%, respectively. There were 15 true negatives and one false positive among the 16 evaluable biopsy sites in the TMB group, with a diagnostic accuracy of 94%.
The diagnostic performance of DSOC (visual impression and targeted biopsy) for the evaluation of the lateral spread of BTC based on endoscopist experience is shown in Table 4. Experts demonstrated higher negative predictive values and diagnostic accuracy with respect to both DSOC visual impression (negative predictive value, 94% vs. 70%, p=0.004; diagnostic accuracy, 92% vs. 73%, p=0.015) and targeted biopsy (negative predictive value, 89% vs. 65%, p=0.008; diagnostic accuracy, 89% vs. 69%, p=0.015) for the evaluation of lateral spread of BTC. The diagnostic accuracy of DSOC visual impression plus targeted biopsy was also higher among experts (93% vs. 80%, p=0.069).
This study evaluated the utility of two techniques, DMB and TMB, for the preoperative evaluation of BTC. The overall adequate tissue acquisition rate for nonstenotic bile duct sites was similar between the two groups (61% vs. 69%, p=0.233), and the adequate tissue acquisition rates for target sites beyond secondary biliary radicles were low in both groups. The diagnostic accuracy of DMB was higher among experts (89%) than among trainees (69%). The diagnostic accuracy among trainees improved to 80% when the DSOC visual impression was added. Although the sample size was limited, TMB showed high diagnostic accuracy regardless of the endoscopist’s expertise.
Endoscopic transpapillary mapping biopsy under fluoroscopy is the standard technique for the evaluation of the lateral spread of BTC. However, this method has several drawbacks, including difficult access to target sites due to the limited maneuverability of biopsy forceps and the inability to perform targeted biopsy under direct visualization. A previous randomized study reported that the accessibility of target sites was only 71% using this conventional technique.15 POCS is a platform that enables targeted biopsy under direct visualization. TMB is another method that is considered optimal for successful access to target sites. Although there are a few studies regarding the utility of DMB,14,15 data regarding the utility of TMB are scarce.18
The technical success rate of reaching the target sites was 95% (39/41) for DMB and 100% (13/13) for TMB in this study. Despite improvements, including the tapered tip and 4-way tip deflection,21,22 it may still be difficult to advance the cholangioscope through stiff biliary strictures. While dilating the stricture before cholangioscopy may be useful when faced with stiff biliary strictures, this was not performed in the two failed cases in this study. As TMB using a 7 Fr-delivery system makes it easier to reach the target sites, it may be an acceptable option when DSOC is unavailable or when DSOC expertise is limited.
With respect to tissue procurement, specimens obtained using DMB are generally smaller than those obtained using conventional biopsies due to the small caliber forceps that are employed.23 Three recent studies reported adequate tissue acquisition rates per biopsy and per biopsy site of about 69% and 78%, respectively.14,15,24 These results were slightly better than those of the current study (61% and 74%), possibly due to the high number of procedures performed by trainees in our study. The adequate tissue acquisition rate per biopsy of DMB was 67% (range, 46%–100%) for nonstenotic bile duct sites proximal to secondary biliary radicles and 44% (range, 0%–52%) for those beyond secondary biliary radicles. Although the optimal number of biopsies for nonstenotic bile duct sites has not been fully established, it may be preferable to conduct multiple biopsies of the same site, given that we only took an average of 1.9 biopsies per site.
On the other hand, TMB can theoretically obtain a larger specimen amount compared to DMB due to the use of larger caliber forceps. A retrospective study involving 50 patients reported that the rate of adequate sampling was 91% using this method.18 In our study, the adequate tissue acquisition rates per biopsy and per biopsy site were 69% and 82%, respectively. The discrepancy between these two studies might be explained by differences in biopsy sites and in the definition of adequate tissue. Adequate tissue was defined as the inclusion of submucosal tissue in the study discussed above, whereas it was defined as the inclusion of biliary epithelium in our study. Most of our inadequate specimens contained only connective or fibrotic tissues and lacked the biliary epithelium. The biliary epithelium may have fallen off the bile duct surface due to cholangitis or previous biliary stenting, which was present in 69% of patients in which TMB was performed. Furthermore, the tissue acquisition rate per biopsy of TMB was 76% (range, 57%–100%) for nonstenotic bile duct sites proximal to secondary biliary radicles and 54% (range, 0%–86%) for those beyond the secondary biliary radicles. Obtaining multiple biopsies per site may also be helpful when conducting TMB.
The sensitivity of targeted biopsy alone was low in previous studies regarding the utility of POCS-guided mapping biopsy.23,25,26 In this study, the overall sensitivity of targeted biopsy alone was very low (45% among experts and 35% among trainees), most likely due to the low adequate tissue acquisition rates. Endoscopists’ expertise affected the diagnostic accuracy of both DSOC visual impression (92% vs. 73%, p=0.015) and targeted biopsy (89% vs. 69%, p=0.015) in this study, replicating a previous study focusing on the diagnosis of indeterminate biliary strictures.16 It is notable that the diagnostic accuracy among trainees improved to 80% when visual impression and targeted biopsy were combined, highlighting the importance of cholangioscopic findings. The severity of hyperbilirubinemia16 and previous biliary stenting might also affect the diagnostic performance of DSOC. These factors could not be evaluated in our study, as the median bilirubin level was within normal limits, and most patients (92%) had received prior biliary stenting. We achieved high diagnostic accuracy in TMB in this study, although a larger sample size would have been desirable.
Our study has several limitations. First, this was a retrospective study from a single institution with a limited sample size; the number of patients who received both TMB and surgical resection was especially small, and potential selection bias might have been present. Second, our cohort was composed of BTCs in various locations, leading to variable biliary stricture severity and mapping biopsy site locations. Third, previous biliary stenting may have led to the loss of biliary epithelium, limiting the evaluation of tissue adequacy and lateral extension as well as the generalizability of this study to intervention-naïve bile ducts. Finally, variations in endoscopist expertise may have affected the outcomes. On the other hand, the last two points may also be considered strengths of this study, allowing it to better reflect real-world clinical practice.
In conclusion, the overall adequate tissue acquisition rate for nonstenotic bile duct sites was similar between the two techniques. Adequate tissue acquisition rates for target sites beyond secondary biliary radicles were low, which may be improved by obtaining multiple biopsies from the same site. Endoscopist expertise was correlated with the diagnostic performance of the DSOC for the evaluation of the lateral spread of BTC. As DMB is expensive and requires expertise, TMB may be an acceptable option when DSOC is unavailable or when DSOC expertise is limited.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest. Conflicts of interest outside this work are as follows: Takashi Sasaki received honoraria from Boston Scientific Japan; Naoki Sasahira received honoraria from Boston Scientific, Gadelius Medical, and consultancies from Gadelius Medical.
REFERENCES
1. Igami T, Nagino M, Oda K, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann Surg. 2009; 249:296–302.
2. Mohamadnejad M, DeWitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011; 73:71–78.
3. Ito K, Sakamoto Y, Isayama H, et al. The impact of MDCT and endoscopic transpapillary mapping biopsy to predict longitudinal spread of extrahepatic cholangiocarcinoma. J Gastrointest Surg. 2018; 22:1528–1537.
4. Watadani T, Akahane M, Yoshikawa T, et al. Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findings. Radiat Med. 2008; 26:402–407.
5. Park HS, Lee JM, Choi JY, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008; 190:396–405.
6. Noda Y, Fujita N, Kobayashi G, et al. Intraductal ultrasonography before biliary drainage and transpapillary biopsy in assessment of the longitudinal extent of bile duct cancer. Dig Endosc. 2008; 20:73–78.
7. Kawakami H, Kuwatani M, Abe Y, et al. A guidewire-assisted biopsy technique to assist advancement through a biliary stricture to perform selective mapping biopsy. Endoscopy. 2015; 47 Suppl 1 UCTN:E217–E218.
8. Hijioka S, Hara K, Mizuno N, et al. A novel technique for endoscopic transpapillary “mapping biopsy specimens” of superficial intraductal spread of bile duct carcinoma (with videos). Gastrointest Endosc. 2014; 79:1020–1025.
9. Hamada T, Takahara N, Nakai Y, et al. The “zipline” technique for endoscopic transpapillary biliary biopsy. Endoscopy. 2020; 52:236–237.
10. Manta R, Frazzoni M, Conigliaro R, et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013; 27:1569–1572.
11. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012; 75:347–353.
12. Gerges C, Beyna T, Tang RS, et al. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: a prospective, randomized, multicenter trial (with video). Gastrointest Endosc. 2020; 91:1105–1113.
13. Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015; 82:608–614.e2.
14. Onoyama T, Hamamoto W, Sakamoto Y, et al. Peroral cholangioscopy-guided forceps mapping biopsy for evaluation of the lateral extension of biliary tract cancer. J Clin Med. 2021; 10:597.
15. Ogawa T, Kanno Y, Koshita S, et al. Cholangioscopy- versus fluoroscopy-guided transpapillary mapping biopsy for preoperative evaluation of extrahepatic cholangiocarcinoma: a prospective randomized crossover study. Surg Endosc. 2021; 35:6481–6488.
16. Jang S, Stevens T, Kou L, et al. Efficacy of digital single-operator cholangioscopy and factors affecting its accuracy in the evaluation of indeterminate biliary stricture. Gastrointest Endosc. 2020; 91:385–393.e1.
17. Ko SW, Lee SS, So H, et al. A novel method of biopsy for indeterminate pancreaticobiliary strictures: tube-assisted biopsy. Endoscopy. 2020; 52:589–594.
18. Okada H, Uza N, Matsumori T, et al. A novel technique for mapping biopsy of bile duct cancer. Endoscopy. 2021; 53:647–651.
19. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
20. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–458.
21. Kulpatcharapong S, Pittayanon R, J Kerr S, et al. Diagnostic performance of different cholangioscopes in patients with biliary strictures: a systematic review. Endoscopy. 2020; 52:174–185.
22. Kanno Y, Koshita S, Ogawa T, et al. Peroral cholangioscopy by SpyGlass DS versus CHF-B260 for evaluation of the lateral spread of extrahepatic cholangiocarcinoma. Endosc Int Open. 2018; 6:E1349–E1354.
23. Nishikawa T, Tsuyuguchi T, Sakai Y, et al. Preoperative assessment of longitudinal extension of cholangiocarcinoma with peroral video-cholangioscopy: a prospective study. Dig Endosc. 2014; 26:450–457.
24. Onoyama T, Takeda Y, Kawata S, et al. Adequate tissue acquisition rate of peroral cholangioscopy-guided forceps biopsy. Ann Transl Med. 2020; 8:1073.
25. Osanai M, Itoi T, Igarashi Y, et al. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: a prospective multicenter study. Endoscopy. 2013; 45:635–642.
26. Ogawa T, Ito K, Koshita S, et al. Usefulness of cholangioscopic-guided mapping biopsy using SpyGlass DS for preoperative evaluation of extrahepatic cholangiocarcinoma: a pilot study. Endosc Int Open. 2018; 6:E199–E204.
Fig. 1.
Mapping biopsy using a biliary stent delivery system (tube-assisted mapping biopsy). (A) A guidewire was advanced into the posterior branch. (B) A 7 Fr-delivery system was inserted into the posterior branch over a guidewire and the pusher catheter remained in the posterior branch after removing the inner catheter and guidewire. A radio-opaque marker was located at the tip of the pusher catheter, which made it easy to adjust the position of the pusher catheter towards the target site. (C) A conventional biopsy forceps with a 1.8-mm diameter cup was advanced through the pusher catheter to the target site under fluoroscopic guidance; biopsy of the posterior branch was performed.
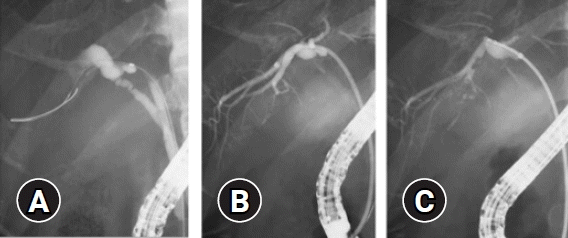
Fig. 2.
Patient flow chart. DSOC, digital single-operator cholangioscopy; BTC, biliary tract cancer.
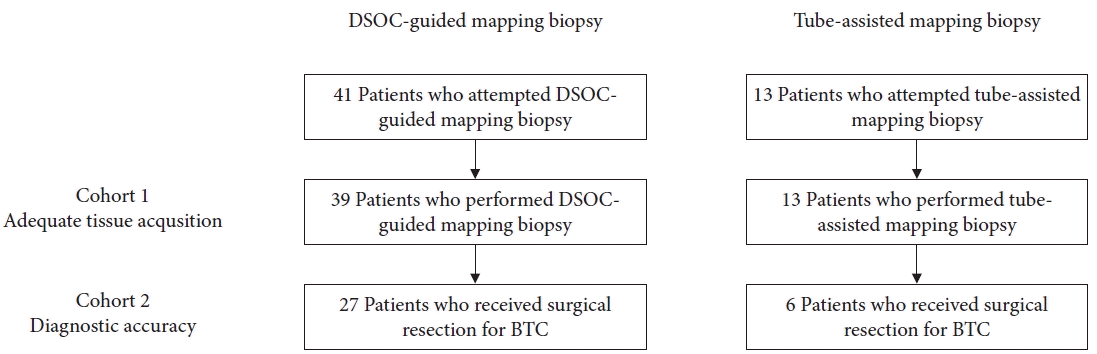
Table 1.
Patient and procedural characteristics
Table 2.
Comparison of adequate tissue acquisition rate per biopsy between DSOC-guided biopsy and tube-assisted biopsy
Table 3.
Pathological characteristics of patients who received surgical resection
Table 4.
Diagnostic performance of DSOC for the evaluation of lateral spread of BTC according to endoscopist experience




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download