Abstract
Background
Postsurgical hypocalcemia is the most common and troublesome consequence of thyroidectomy. We investigated the potential role of routine calcium or vitamin D supplementation in preventing postsurgical hypocalcemia.
Methods
We searched MEDLINE and Embase for English-language publications using the keywords “calcium,” “vitamin D,” and “thyroid cancer.” The primary outcome was any postoperative hypocalcemia, and the secondary outcome was symptomatic hypocalcemia.
Results
Four studies that included 381 patients were eligible for this meta-analysis. A random-effects model showed no significant difference in the occurrence of hypocalcemia between calcium/vitamin D treatment and placebo/no treatment. However, the occurrence of symptomatic hypocalcemia was lower in patients with calcium/vitamin D treatment. In the combined results, preoperative calcium and vitamin D supplementation were associated with a reduced incidence of symptomatic hypocalcemia.
Thyroid cancer is the most common type of malignant endocrine cancer, and its incidence is continuing to rise worldwide [1]. Total thyroidectomy followed by radioactive iodine treatment together with life-long administration of thyroid hormone is the treatment strategy for most patients with thyroid cancer [2]. Total thyroidectomy is one of the most common endocrine-gland operations and most patients recover fully without any adverse events [3]. Hypoparathyroidism (hypoPTH) and hypocalcemia are the most common and troublesome long-term consequence of bilateral and reoperative thyroid operations [4,5]. Large epidemiologic studies have estimated that the overall incidence of hypoPTH after anterior neck surgery is approximately 8%, with 75% of cases resolving within 6 months and the remaining 25% resulting in permanent hypoPTH [6]. Postsurgical hypocalcemia impacts negatively of patient’s quality of life due to the need for lifetime medication, regular visits and significant long-term costs. Most transient hypocalcemias may not warrant long‑term calcium and vitamin D supplementation [3]. The most common early symptoms of postsurgical hypocalcemia are paresthesias, or numbness and tingling, of the perioral region and the fingertips. More sustained muscle contraction may lead to laryngospasm, and more severe neural excitability may lead to seizures. Moreover, severe hypocalcemia can cause life-threatening complications, such as laryngospasm and cardiac arrhythmias [4,7]. Therefore, close monitoring of calcium and parathyroid hormone (PTH) levels is indicated in order to identify hypoPTH before the development of severe, symptomatic hypocalcemia after surgery [6].
An empirical prophylactic approach for managing potential post-thyroidectomy hypocalcemia is to prescribe oral calcium routinely with or without oral calcitriol, without testing PTH or calcium levels [8,9]. This approach is cost-effective, is not labor intensive, is expeditious, and can hasten hospital discharge after thyroidectomy [4]. Previous studies have shown the efficacy of routine postsurgical calcium and vitamin D supplementation as a prophylactic strategy to prevent hypocalcemia in patients undergoing total thyroidectomy [10-12], but the effectiveness of preoperative supplementation is limited. Moreover, there is no consensus on the role of routine calcium and/or vitamin D before thyroid surgery [11].
Our objectives in this meta-analysis are to evaluate the potential role of routine calcium and vitamin D supplementation for the prevention of postsurgical hypocalcemia and to draw therapy guidelines that may prevent this common complication.
We did a systematic search for studies from MEDLINE (inception to March 2019) and Embase (inception to March 2019) using the keywords “calcium” and “thyroid cancer” or “vitamin D” and “thyroid cancer.” The primary analysis included studies comparing the occurrence of postsurgical hypocalcemia in subjects with thyroid cancer who had preoperative calcium or vitamin D treatment. Manual searches included scanning of reference lists for relevant studies and eligible articles. We included articles written in English only in this study. Two of us conducted the search of our own, and disagreements were settled by discussion.
We extracted data from the publications and recorded the following information independently: first author, year of publication, country, data source, the number of patients and centers enrolled in the study, dosage of calcium or vitamin D, and definition of hypocalcemia. For the outcome data, we calculated the odds ratios (ORs) from the number of cases in which hypocalcemia occurred in each group.
The primary outcome was any postoperative hypocalcemia, and the secondary outcome was symptomatic hypocalcemia. We analyzed data from each study using Review Manager (RevMan, version 5.2, Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). We reported outcome measures as ORs and 95% confidence intervals (CIs), using a fixed-effect or random-effects model according to Mantel and Haenszel, and presented the results as forest plots. An OR greater than 1 implied more hypocalcemia for preoperative calcium or vitamin D users, whereas an OR less than 1 implied less hypocalcemia for such users. Heterogeneity of the studies were assessed using the chi-square test of heterogeneity; I2 >50% was considered significant heterogeneity, as described by Higgins et al. [13]. A p<0.05 was considered to indicate statistical significance.
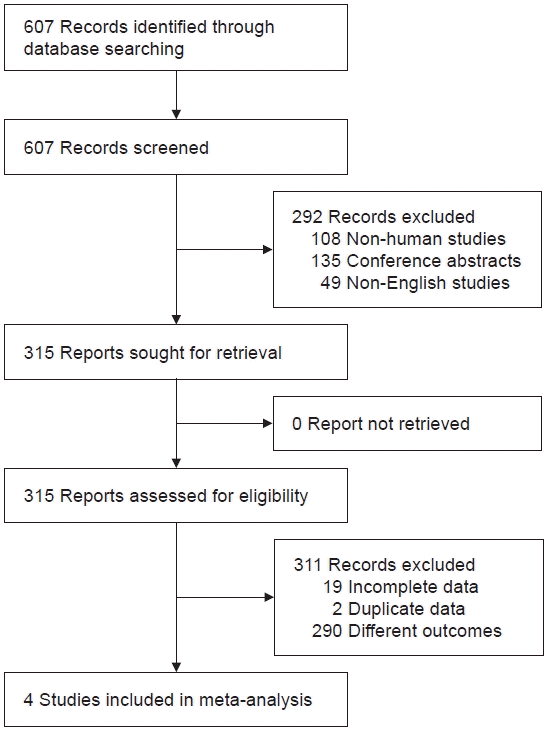
We identified 607 articles through the database. After excluding conference abstracts (n=135), non-human studies (n=108), and non-English studies (n=49), we assessed 315 abstracts for eligibility. After reviewing full-text articles, four studies that included 381 patients were eligible for this analysis [14-17]. The details of the study selection process are depicted as a flowchart (Fig. 1). The summary of studies included is presented in Table 1.
Three studies covering 306 patients among four studies were included in analyzing postoperative hypocalcemia. The random-effects model showed no significant difference in the occurrence of hypocalcemia between calcium/vitamin D treatment and placebo/no treatment (OR, 0.82; 95% CI, 0.36–1.86; I2=60%, p=0.63) (Fig. 2).
Three studies covering 275 patients among four studies were included in analyzing postoperative hypocalcemia. The occurrence of symptomatic hypocalcemia was lower in patients with calcium/vitamin D treatment than in those with placebo/no treatment (OR, 0.44; 95% CI, 0.22–0.88; I2=41%, p=0.02) (Fig. 3).
The mainstay of therapy for differentiated thyroid cancer is thyroidectomy [2]. Postsurgical hypocalcemia following thyroidectomy is a common complication because of damage to the parathyroid glands. It also increases healthcare‐associated expenditure because of increased monitoring requirements, pharmacotherapy, and prolonged hospitalization in addition to patient morbidity, Interventions that minimize postsurgical hypocalcemia are needed in order to improve patient care and waste of resources. In combined results, we found that preoperative calcium and vitamin D supplementation was associated with a reduced incidence of symptomatic hypocalcemia after total thyroidectomy.
The mechanisms of postsurgical hypoPTH are related to disruption of parathyroid arterial supply or venous drainage, mechanical, thermal or electrical injury, and partial or complete removal [18]. Therefore, the most straightforward way to avoid hypoPTH is to limit the extent of thyroidectomy to a unilateral approach [4]. The best prophylaxis to avoid postsurgical hypocalcemia after total thyroidectomy is parathyroid gland preservation during operation to preserve the blood supply to the parathyroid glands [19]. Even when these glands are thought to be well preserved during surgery, normal postsurgical parathyroid function is not guaranteed [20].
Several interventions to reduce the incidence of postsurgical hypocalcemia have been suggested because patients with symptomatic hypocalcemia undergoes physical and mental suffering [12]. Therefore, routine calcium and vitamin D supplementation is advocated in many clinical centers [4,19]. These prophylactic approaches to prevent postsurgical hypocalcemia is to routine prescription of oral calcium with or without calcitriol [8,9]. Typically, oral calcium carbonate is the most widely available and inexpensive preparation and is given as 500–625 mg to 1,000–1,250 mg two to three times a day. This routine administration of oral calcium is known to reduce postsurgical hypocalcemia to approximately 10% [21]. Because of low cost and ease of dosing for patients at risk for hypoPTH, we also recommend universal calcium prophylaxis like others [19].
Vitamin D deficiency is an independent risk factor of postsurgical hypocalcemia [5]. Moreover, the severity of hypocalcemia seems to be remarkably higher in those with lower than normal preoperative vitamin D levels [22,23]. Therefore, prophylactic treatment of hypocalcemia with vitamin D and calcium is a reasonable strategy with synergy [24]. Impaired PTH secretion with inhibition of bone resorption, reduction of vitamin D synthesis by the kidneys, and reduced intestinal absorption of calcium results in postoperative hypocalcemia [25]. Adding calcitriol (1,25-(OH)2-D3) adds to the cost but increases the effectiveness of oral calcium. It also increases calcium absorption and increasing intestinal calcium transport into the blood. This effect on calcium absorption usually takes few days [14]. Therefore, preoperative supplementation in patients undergoing thyroidectomy with calcitriol are expected to increase the efficacy of routine calcium supplementation in the immediate postsurgical period, thereby decreasing the duration of transient hypocalcemia [4].
PTH level has also been suggested as a reliable marker of postsurgical permanent hypoPTH [26]. However, development of acute hypocalcemia after thyroid surgery lags behind the decline in the serum PTH level, and the patient may have been from the hospital before their serum calcium having reaches a nadir, which may occur 24 to 72 hours after thyroidectomy [4]. Therefore, it is important to anticipate the possibility of progressive hypocalcemia, to educate patients about its possible development and steps they should take to avoid and treat it, and to institute preemptive measures that both prevent and correct hypocalcemia in the presurgical period.
Postoperative calcium plus vitamin D is known to be effective in preventing postoperative hypocalcemia and decreasing the demand for intravenous calcium supplementation [12]. This meta-analysis found the role of preoperative oral calcium and vitamin D supplementation in avoiding postsurgical symptomatic hypocalcemia. This prophylactic approach may cause uncommon but serious risks of overshooting and causing hypercalcemia and potential renal injury. Therefore, biochemical monitoring for medication tapering is mandatory [4]. However, the half-life of calcitriol is relatively short (5–8 hours), and toxicity from excessive calcitriol ingestion may be reversed quickly (within days) [4].
Our study has some limitations. First, the studies included in the meta-analysis were heterogeneous. There was a high heterogeneity especially among the studies including those at a young age. There is no universal agreement on standardized definitions for postsurgical hypocalcemia and hypoPTH after total thyroidectomy [11]. The reported incidence of postsurgical hypoPTH varies differs greatly, and previous research also suggested that the definition of hypoPTH is not universal throughout the literature [11]. Second, attempting to compare data from the surgical series is difficult and may be inaccurate. Analysis was even more difficult by the diversity of postsurgical electrolyte supplementation protocols used by different doctors. Third, we cannot define the adequate dosage and duration of calcium and vitamin D intake before surgery, because the regimens of each study studies were quite variable very different. Lastly, small numbers of studies included in this meta-analysis might be exposed to publication bias.
Postsurgical hypocalcemia is the most common complication of total thyroidectomy. Increased recognition and early implementation of various strategies can improve clinical outcomes and quality of life. We support the use of preoperative calcium and vitamin D supplementation in conjunction with routine postsurgical supplementation for patients after total thyroidectomy.
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016; 388:2783–95.
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
3. Dedivitis RA, Aires FT, Cernea CR. Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg. 2017; 25:142–6.
4. Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ 3rd, Shaha AR, Shindo ML, et al. American Thyroid Association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid. 2018; 28:830–41.
5. Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. 2014; 101:307–20.
6. Gafni RI, Collins MT. Hypoparathyroidism. N Engl J Med. 2019; 380:1738–47.
7. Bilezikian JP, Brandi ML, Cusano NE, Mannstadt M, Rejnmark L, Rizzoli R, et al. Management of hypoparathyroidism: present and future. J Clin Endocrinol Metab. 2016; 101:2313–24.
8. Singer MC, Bhakta D, Seybt MW, Terris DJ. Calcium management after thyroidectomy: a simple and cost-effective method. Otolaryngol Head Neck Surg. 2012; 146:362–5.
9. Wang TS, Cheung K, Roman SA, Sosa JA. To supplement or not to supplement: a cost-utility analysis of calcium and vitamin D repletion in patients after thyroidectomy. Ann Surg Oncol. 2011; 18:1293–9.
10. Sanabria A, Dominguez LC, Vega V, Osorio C, Duarte D. Routine postoperative administration of vitamin D and calcium after total thyroidectomy: a meta-analysis. Int J Surg. 2011; 9:46–51.
11. Harslof T, Rolighed L, Rejnmark L. Huge variations in definition and reported incidence of postsurgical hypoparathyroidism: a systematic review. Endocrine. 2019; 64:176–83.
12. Xing T, Hu Y, Wang B, Zhu J. Role of oral calcium supplementation alone or with vitamin D in preventing post-thyroidectomy hypocalcaemia: a meta-analysis. Medicine (Baltimore). 2019; 98:e14455.
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
14. Maxwell AK, Shonka DC Jr, Robinson DJ, Levine PA. Association of preoperative calcium and calcitriol therapy with postoperative hypocalcemia after total thyroidectomy. JAMA Otolaryngol Head Neck Surg. 2017; 143:679–84.
15. Jaan S, Sehgal A, Wani RA, Wani MA, Wani KA, Laway BA. Usefulness of pre-and post-operative calcium and vitamin D supplementation in prevention of hypocalcemia after total thyroidectomy: a randomized controlled trial. Indian J Endocrinol Metab. 2017; 21:51–5.
16. Rowe CW, Arthurs S, O'Neill CJ, Hawthorne J, Carroll R, Wynne K, et al. High-dose preoperative cholecalciferol to prevent post-thyroidectomy hypocalcaemia: a randomized, double-blinded placebo-controlled trial. Clin Endocrinol (Oxf). 2019; 90:343–50.
17. Yu YR, Fallon SC, Carpenter JL, Athanassaki I, Brandt ML, Wesson DE, et al. Perioperative determinants of transient hypocalcemia after pediatric total thyroidectomy. J Pediatr Surg. 2017; 52:684–8.
18. Anastasiou OE, Yavropoulou MP, Papavramidis TS, Tzouvara C, Triantafyllopoulou K, Papavramidis S, et al. Secretory capacity of the parathyroid glands after total thyroidectomy in normocalcemic subjects. J Clin Endocrinol Metab. 2012; 97:2341–6.
19. Stack BC Jr, Bimston DN, Bodenner DL, Brett EM, Dralle H, Orloff LA, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism--definitions and management. Endocr Pract. 2015; 21:674–85.
20. Park I, Rhu J, Woo JW, Choi JH, Kim JS, Kim JH. Preserving parathyroid gland vasculature to reduce post-thyroidectomy hypocalcemia. World J Surg. 2016; 40:1382–9.
21. Roh JL, Park JY, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer. 2009; 115:251–8.
22. Erbil Y, Barbaros U, Temel B, Turkoglu U, Issever H, Bozbora A, et al. The impact of age, vitamin D(3) level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am J Surg. 2009; 197:439–46.
23. Kim WW, Chung SH, Ban EJ, Lee CR, Kang SW, Jeong JJ, et al. Is preoperative vitamin D deficiency a risk factor for postoperative symptomatic hypocalcemia in thyroid cancer patients undergoing total thyroidectomy plus central compartment neck dissection? Thyroid. 2015; 25:911–8.
24. Al Khadem MG, Rettig EM, Dhillon VK, Russell JO, Tufano RP. Postoperative IPTH compared with IPTH gradient as predictors of post-thyroidectomy hypocalcemia. Laryngoscope. 2018; 128:769–74.
25. Shoback D. Clinical practice. Hypoparathyroidism N Engl J Med. 2008; 359:391–403.
26. Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery. 2002; 131:515–20.
Fig. 2.
Forest plot of any postoperative hypocalcemia. Ca, calcium; Vit.D, vitamin D; CI, confidence interval; M-H, Mantel and Haenszel.
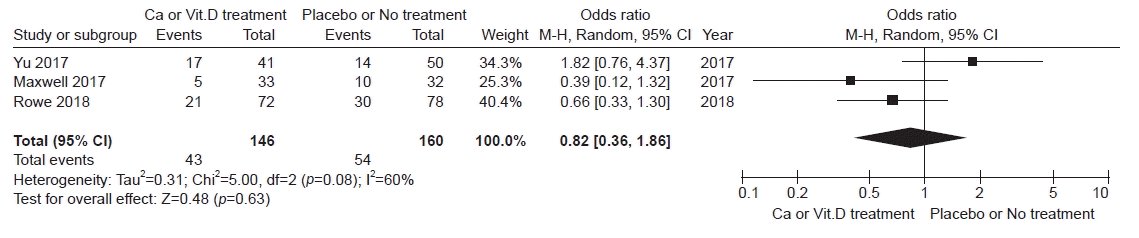
Fig. 3.
Forest plot of symptomatic hypocalcemia. Ca, calcium; Vit.D, vitamin D; CI, confidence interval; M-H, Mantel and Haenszel.
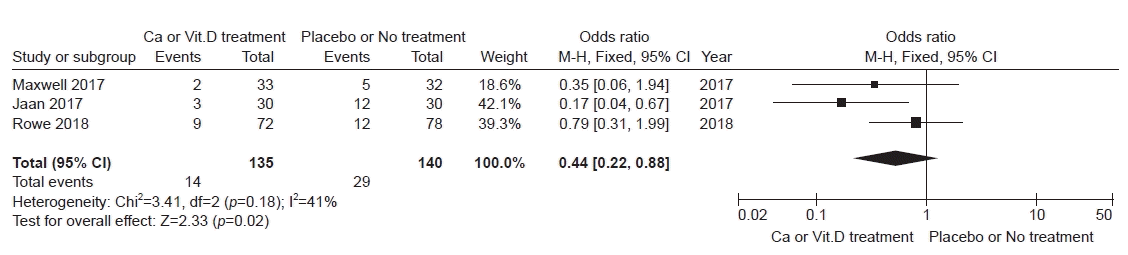
Table 1.
Study characteristics
| Author (year) | Country | No. of subjects |
Dosage |
Definition of hypocalcemia |
||||
|---|---|---|---|---|---|---|---|---|
| Patient | Control | Calcium | Vitamin D | Placebo/no treatment | Any postoperative | Symptomatic | ||
| Rowe et al. (2018) [16] | Australia | 72 | 78 | - | 6 Gelatin capsules of cholecalciferol (6×50,000 IU) | 6 Capsules of rice flour | Corrected serum calcium <2.10 mmol/L) at any time point during the first 180 postoperative days | Any one of: perioral paresthesia, tetany, positive Chvostek’s sign or prolonged QT‐interval on ECG associated with biochemical hypocalcemia |
| Maxwell et al. (2017) [14] | USA | 33 | 32 | 5 Days of preoperative oral calcium carbonate, 1,000–1,500 mg, 3 times daily and calcitriol, 0.25–0.5 μg twice daily supplementation | - | NA | Postoperative calcium levels and the development of postoperative hypocalcemia (calcium levels <8.0 mg/dL) at 24 hours | Calcium level <8 mg/dL with symptoms |
| Yu et al. (2017) [17] | USA | 41 | 50 | NA | - | NA | Calcium <8.0 mg/dL or ionized calcium <1.0 mmol/L | - |
| Jaan et al. (2017) [15] | India | 30 | 30 | Oral calcium 500 mg every 6 hours and calcitriol 0.25 μg every 6 hours (Shelcal CT) starting 7 days before surgery and continued for 7 days postoperatively | - | - | - | Paresthesia of fingertips and perioral area, tetany, neuropsychiatric manifestations, Chvostek and Trousseau signs, and electrocardiogram evidence of prolonged corrected QT interval by Bazett’s formula |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download