1. Jonas RA. Training fellows in paediatric cardiac surgery. Cardiol Young. 2016; 26(8):1474–1483. PMID:
28148328.

2. Halsted WS. The training of the surgeon. Bull Johns Hopkins Hosp. 1904; 15:267–275.
3. Barnes RW, Lang NP, Whiteside MF. Halstedian technique revisited. Innovations in teaching surgical skills. Ann Surg. 1989; 210(1):118–121. PMID:
2742408.
4. DaRosa DA, Zwischenberger JB, Meyerson SL, George BC, Teitelbaum EN, Soper NJ, et al. A theory-based model for teaching and assessing residents in the operating room. J Surg Educ. 2013; 70(1):24–30. PMID:
23337666.

5. George BC, Teitelbaum EN, Meyerson SL, Schuller MC, DaRosa DA, Petrusa ER, et al. Reliability, validity, and feasibility of the Zwisch scale for the assessment of intraoperative performance. J Surg Educ. 2014; 71(6):e90–e96. PMID:
25192794.

6. Karl TR, Jacobs JP. Paediatric cardiac surgical education: which are the important elements? Cardiol Young. 2016; 26(8):1465–1470. PMID:
28148329.

7. Kenny L, Booth K, Freystaetter K, Wood G, Reynolds G, Rathinam S, et al. Training cardiothoracic surgeons of the future: the UK experience. J Thorac Cardiovasc Surg. 2018; 155(6):2526–2538.e2. PMID:
29661505.

8. Sadaba JR, Urso S. Does the introduction of duty-hour restriction in the United States negatively affect the operative volume of surgical trainees? Interact Cardiovasc Thorac Surg. 2011; 13(3):316–319. PMID:
21719511.

9. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014; 186(10):761–765. PMID:
24847138.

10. Moffatt-Bruce SD, Ross P, Williams TE Jr. American Board of Thoracic Surgery examination: fewer graduates, more failures. J Thorac Cardiovasc Surg. 2014; 147(5):1464–1470. PMID:
24521972.

11. Kogon B, Karamlou T, Baumgartner W, Merrill W, Backer C. Congenital cardiac surgery fellowship training: a status update. J Thorac Cardiovasc Surg. 2016; 151(6):1488–1495. PMID:
27002229.

12. Fraser CD. Becoming a congenital heart surgeon in the current era: realistic expectations. J Thorac Cardiovasc Surg. 2016; 151(6):1496–1497. PMID:
27016794.

13. Hicks GL Jr, Brown JW, Calhoon JH, Merrill WH. You never know unless you try. J Thorac Cardiovasc Surg. 2008; 136(4):814–815. PMID:
18954616.

14. Tchervenkov CI, Herbst C, Jacobs JP, Al-Halees Z, Edwin F, Dearani JA, et al. Current status of training and certification for congenital heart surgery around the world: proceedings of the meetings of the Global Council on Education for Congenital Heart Surgery of the World Society for Pediatric and Congenital Heart Surgery. World J Pediatr Congenit Heart Surg. 2021; 12(3):394–405. PMID:
33942697.

15. Morales DL, Khan MS, Turek JW, Biniwale R, Tchervenkov CI, Rush M, et al. Report of the 2015 Society of Thoracic Surgeons congenital heart surgery practice survey. Ann Thorac Surg. 2017; 103(2):622–628. PMID:
27553498.
16. Hussein N, Zientara A, Gollmann-Tepeköyly C, Loubani M. Is it time to incorporate hands-on simulation into the cardiothoracic surgery curriculum? Interact Cardiovasc Thorac Surg. 2022; 34(4):564–565. PMID:
34718593.

17. Abdel Shafi AM, Sheikh AM, Awad WI. Comparison of cardiothoracic surgical training before and during the COVID-19 pandemic in the United Kingdom. JTCVS Open. 2021; 7:394–410. PMID:
34308383.

18. Mitzman B. Commentary: A new “lost” generation. JTCVS Open. 2021; 7:411–412. PMID:
34401860.

19. Carpenter AJ, Yang SC, Uhlig PN, Colson YL. Envisioning simulation in the future of thoracic surgical education. J Thorac Cardiovasc Surg. 2008; 135(3):477–484. PMID:
18329455.

20. Trehan K, Kemp CD, Yang SC. Simulation in cardiothoracic surgical training: where do we stand? J Thorac Cardiovasc Surg. 2014; 147(1):18–24.e2. PMID:
24331908.

21. Feins RH, Burkhart HM, Conte JV, Coore DN, Fann JI, Hicks GL Jr, et al. Simulation-based training in cardiac surgery. Ann Thorac Surg. 2017; 103(1):312–321. PMID:
27570162.

22. Ribeiro IB, Ngu JM, Lam BK, Edwards RA. Simulation-based skill training for trainees in cardiac surgery: a systematic review. Ann Thorac Surg. 2018; 105(3):972–982. PMID:
29248416.

23. Yoo SJ, Spray T, Austin EH 3rd, Yun TJ, van Arsdell GS. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. 2017; 153(6):1530–1540. PMID:
28268011.

24. Hussein N, Honjo O, Haller C, Hickey E, Coles JG, Williams WG, et al. Hands-on surgical simulation in congenital heart surgery: literature review and future perspective. Semin Thorac Cardiovasc Surg. 2020; 32(1):98–105. PMID:
31220532.

25. Hussein N, Lim A, Honjo O, Haller C, Coles JG, Van Arsdell G, et al. Development and validation of a procedure-specific assessment tool for hands-on surgical training in congenital heart surgery. J Thorac Cardiovasc Surg. 2020; 160(1):229–240.e1. PMID:
31973896.

26. Hussein N, Honjo O, Haller C, Coles JG, Hua Z, Van Arsdell G, et al. Quantitative assessment of technical performance during hands-on surgical training of the arterial switch operation using 3-dimensional printed heart models. J Thorac Cardiovasc Surg. 2020; 160(4):1035–1042. PMID:
31983523.

27. Hussein N, Honjo O, Barron DJ, Haller C, Coles JG, Van Arsdell G, et al. Assessment tool validation and technical skill improvement in the simulation of the Norwood operation using three-dimensional-printed heart models. Eur J Cardiothorac Surg. 2020; ezaa321. PMID:
33249480.
28. Hussein N, Honjo O, Barron DJ, Haller C, Coles JG, Yoo SJ. The incorporation of hands-on surgical training in a congenital heart surgery training curriculum. Ann Thorac Surg. 2021; 112(5):1672–1680. PMID:
33307072.

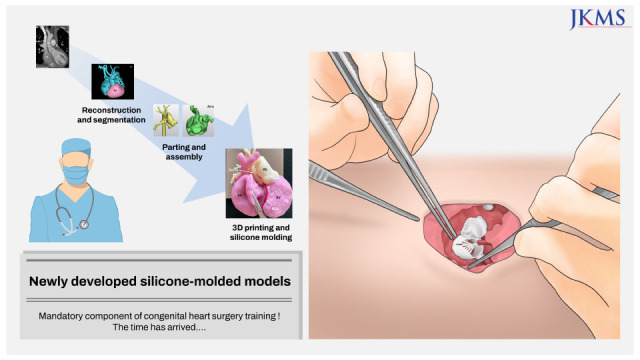
29. Peel B, Lee W, Hussein N, Yoo SJ. State-of-the-art silicone molded models for simulation of arterial switch operation: Innovation with parting-and-assembly strategy. JTCVS Tech. 2022; 12:132–142. PMID:
35403031.

30. Fann JI, Sullivan ME, Skeff KM, Stratos GA, Walker JD, Grossi EA, et al. Teaching behaviors in the cardiac surgery simulation environment. J Thorac Cardiovasc Surg. 2013; 145(1):45–53. PMID:
23098747.
31. Fann JI, Feins RH, Hicks GL Jr, Nesbitt JC, Hammon JW, Crawford FA Jr, et al. Evaluation of simulation training in cardiothoracic surgery: the Senior Tour perspective. J Thorac Cardiovasc Surg. 2012; 143(2):264–272. PMID:
22075060.

32. Kelly JJ, Han JJ, Patrick WL, Mays JC, Iyengar A, Helmers MR, et al. Do-it-yourself simulators and building a culture of practice in the virtual era. JTCVS Tech. 2021; 8:100–111. PMID:
34401826.

33. Hussein N, Van den Eynde J, Callahan C, Guariento A, Gollmann-Tepeköylü C, Elbatarny M, et al. The use of objective assessments in the evaluation of technical skills in cardiothoracic surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2022; 35(3):ivac194. PMID:
35900153.

34. Burkhart HM. Simulation in congenital cardiac surgical education: we have arrived. J Thorac Cardiovasc Surg. 2017; 153(6):1528–1529. PMID:
28359578.

35. Nam JG, Lee W, Jeong B, Park EA, Lim JY, Kwak Y, et al. Three-dimensional printing of congenital heart disease models for cardiac surgery simulation: evaluation of surgical skill improvement among inexperienced cardiothoracic surgeons. Korean J Radiol. 2021; 22(5):706–713. PMID:
33543844.
36. Sadeghi AH, Mathari SE, Abjigitova D, Maat AP, Taverne YJ, Bogers AJ, et al. Current and future applications of virtual, augmented, and mixed reality in cardiothoracic surgery. Ann Thorac Surg. 2022; 113(2):681–691. PMID:
33347848.

37. Arjomandi Rad A, Vardanyan R, Thavarajasingam SG, Zubarevich A, Van den Eynde J, Sá MP, et al. Extended, virtual and augmented reality in thoracic surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2022; 34(2):201–211. PMID:
34542639.

38. Cates CU, Gallagher AG. The future of simulation technologies for complex cardiovascular procedures. Eur Heart J. 2012; 33(17):2127–2134. PMID:
22733836.

39. Tandon A, Burkhardt BE, Batsis M, Zellers TM, Velasco Forte MN, Valverde I, et al. Sinus venosus defects: anatomic variants and transcatheter closure feasibility using virtual reality planning. JACC Cardiovasc Imaging. 2019; 12(5):921–924. PMID:
30553676.
40. Valdis M, Chu MW, Schlachta C, Kiaii B. Evaluation of robotic cardiac surgery simulation training: a randomized controlled trial. J Thorac Cardiovasc Surg. 2016; 151(6):1498–1505.e2. PMID:
26964911.

41. Ramphal PS, Coore DN, Craven MP, Forbes NF, Newman SM, Coye AA, et al. A high fidelity tissue-based cardiac surgical simulator. Eur J Cardiothorac Surg. 2005; 27(5):910–916. PMID:
15848335.

42. Reuthebuch O, Lang A, Groscurth P, Lachat M, Turina M, Zünd G. Advanced training model for beating heart coronary artery surgery: the Zurich heart-trainer. Eur J Cardiothorac Surg. 2002; 22(2):244–248. PMID:
12142193.

43. Fann JI, Calhoon JH, Carpenter AJ, Merrill WH, Brown JW, Poston RS, et al. Simulation in coronary artery anastomosis early in cardiothoracic surgical residency training: the Boot Camp experience. J Thorac Cardiovasc Surg. 2010; 139(5):1275–1281. PMID:
19846125.

44. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013; 74(2):664–670. PMID:
23354267.
45. Yoo SJ, Thabit O, Kim EK, Ide H, Yim D, Dragulescu A, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. 2015; 2(1):3. PMID:
30050975.

46. Hoashi T, Ichikawa H, Nakata T, Shimada M, Ozawa H, Higashida A, et al. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact Cardiovasc Thorac Surg. 2018; 27(5):749–755. PMID:
29846596.

47. Peel B, Voyer-Nguyen P, Honjo O, Yoo SJ, Hussein N. Development of a dynamic Chest Wall and operating table simulator to enhance congenital heart surgery simulation. 3D Print Med. 2020; 6(1):12. PMID:
32488567.

48. Yoo SJ, Hussein N, Peel B, Coles J, van Arsdell GS, Honjo O, et al. 3D modeling and printing in congenital heart surgery: entering the stage of maturation. Front Pediatr. 2021; 9:621672. PMID:
33614554.

49. Hussein N, Voyer-Nguyen P, Portnoy S, Peel B, Schrauben E, Macgowan C, et al. Simulation of semilunar valve function: computer-aided design, 3D printing and flow assessment with MR. 3D Print Med. 2020; 6(1):2. PMID:
32016687.

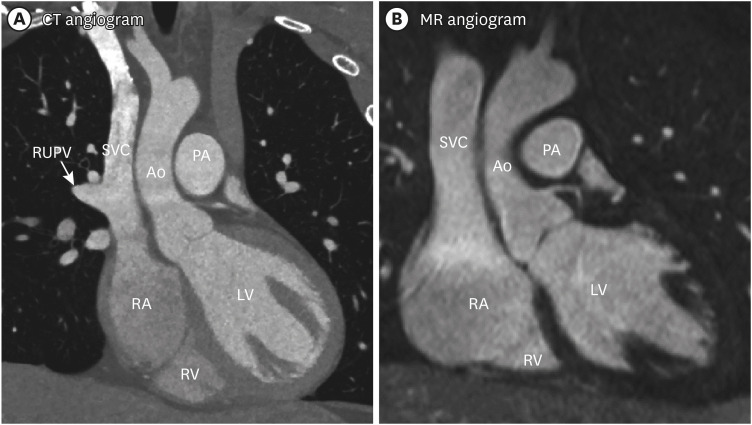
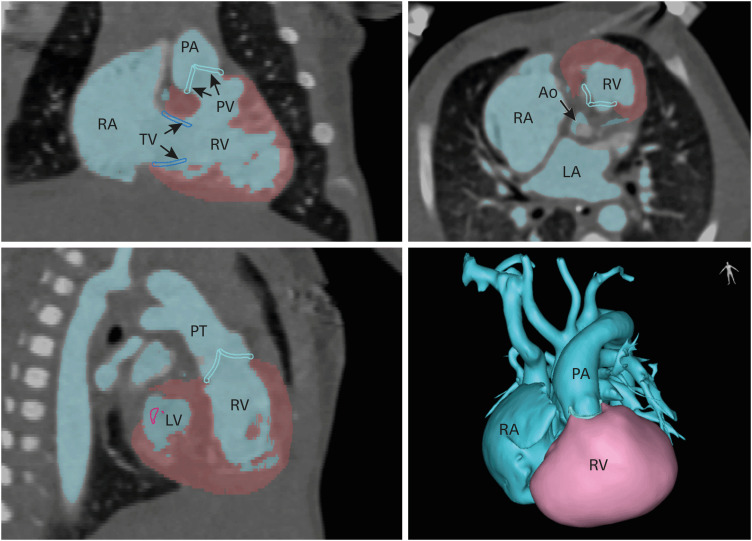
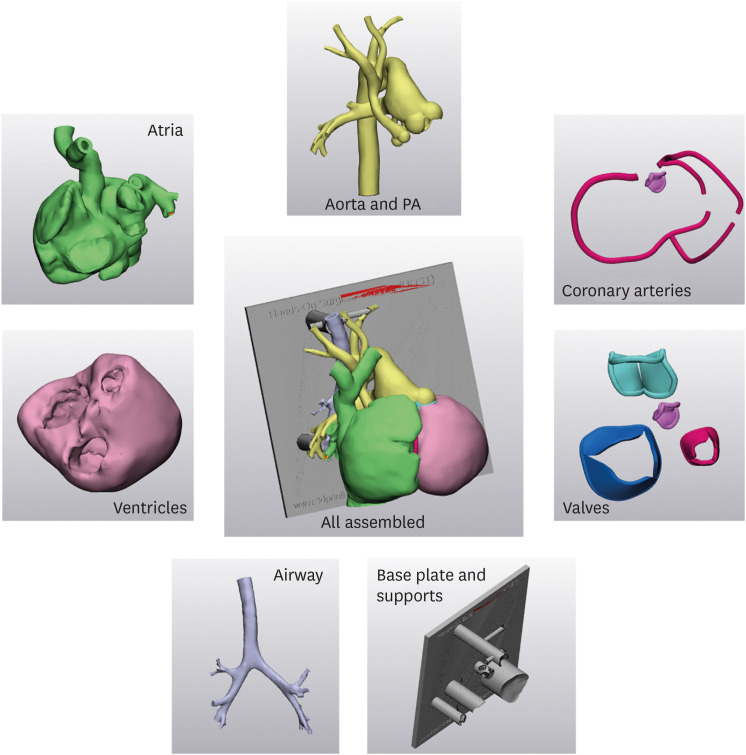
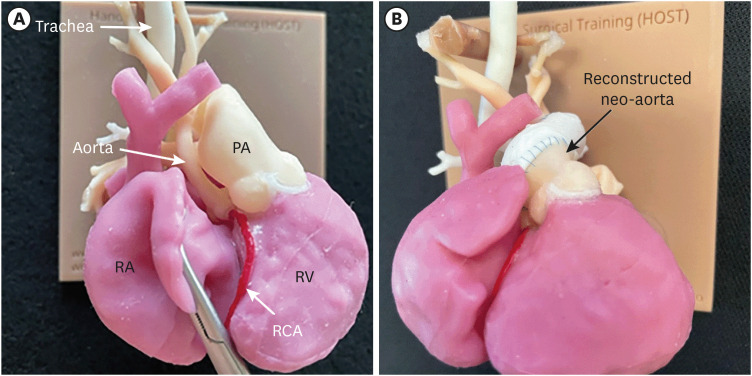




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download