Abstract
Background
Although recent studies comparing various dosages and intervals of vitamin D supplementation have been published, it is yet to be elucidated whether there is an appropriate dose or interval to provide benefit regarding fracture risk. We aimed to assess the published evidence available to date regarding the putative beneficial effects of vitamin D supplements on fractures and falls according to various dosages and intervals.
Methods
We performed a meta-analysis of randomized controlled studies reporting associations between vitamin D supplementation and the risks of fractures and falls in PubMed, EMBASE, and Cochrane library. Studies with supplements of ergocalciferol or calcitriol, those with a number of event ≤10, or those with a follow-up duration of less than 6 months were also excluded.
Results
Thirty-two studies were included in the final analysis. Vitamin D supplementation with daily dose of 800 to 1,000 mg was associated with lower risks of osteoporotic fracture and fall (pooled relative risk [RR], 0.87; 95% confidence interval [CI], 0.78 to 0.97 and RR, 0.91; 95% CI, 0.85 to 0.98), while studies with <800 or >1,000 mg/day did not. Also, among intervals, daily administration of vitamin D was associated with the reduced risk of falls, while intermittent dose was not. Also, patients with vitamin D deficiency showed a significant risk reduction of falls after vitamin D supplementation.
Vitamin D has been known to be vital to musculoskeletal health since it promotes mineralization of osteoid tissue and supports calcium homeostasis and muscle function [1-3]. In previous studies, vitamin D deficiency was associated with low bone mineral density and increased fracture risk in longitudinal studies [4,5]. Vitamin D deficiency was also associated with decreased muscle mass and strength, supporting the potential benefits of vitamin D supplementation [2,4]. However, the optimal ways to administer vitamin D supplementation to prevent fractures have been debated until recently [6,7].
Contrary to expectations, the effect of vitamin D supplementation on fracture or fall risk was inconsistent or neutral, especially in the community-dwelling population [6]. In current guidelines, 800 IU/day of vitamin D with calcium supplementation has been recommended in older adults with vitamin D deficiency or those who are institutionalized [8,9]. Nonetheless, in a recent meta-analysis, treatment with vitamin D did not affect the incidence of fractures or falls among asymptomatic, community-dwelling populations with low vitamin D levels [6]. However, given that physicians have various options for vitamin D supplements in various doses, intervals, and oral/injectable forms, it is yet to be elucidated whether there is an appropriate dose or interval to benefit fracture risk. Subsequently, in a recent year, studies with various dosages and intervals have been published to address this question [7,10]. Since the dosage and interval of vitamin D supplementation are essential in assessing the effects on musculoskeletal outcomes, updated guidance on the optimal doses and dosing schedules for preventing fractures and falls is needed.
Therefore, the meta-analysis aimed to assess the published evidence available to date regarding the putative beneficial effects of vitamin D supplements on fractures and falls according to various dosages and intervals.
We searched PubMed, Embase, and Cochrane Library databases using keywords related to vitamin D supplementation with cholecalciferol, fractures, falls, and a randomized controlled study published until March 30, 2021. Peer Review of Electronic Search Strategies to design a structural search strategy were done (Supplemental Methods) [11]. Also, a manual search was conducted using study identifiers or references from previous studies. The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12] and meta-analyses of observational studies in epidemiology [13]. The PRISMA checklist is available from Supplemental Table S1 [14], and the protocol for this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO ID 246065).
The studies were selected using the PRISMA flow diagram [12]. After removing duplicates, the titles and abstracts were screened to identify eligible studies for full-text review. Studies with ≤10 patients with fractures or falls were excluded because the calculations of mean and standard deviations (SDs) were considered unreliable in these studies. Studies comparing vitamin D supplements with placebo or vitamin D supplements of dose <400 IU/day were selected. The authors were contacted to provide organized results when data were not presented according to fracture or fall status. Studies using ergocalciferol or calcitriol or those with a follow-up duration of less than 6 months were also excluded. We collected article information from each study, including the authors’ details, study design, location, intervention, follow-up period, and study outcome. In addition, patient characteristics were collected, including sex, age, and study settings (community-based or institutionalized). In the subgroup analysis, studies were categorized according to a daily vitamin D dose of <800, 800 to 1,000, and >1,000 IU/day. In addition, according to the administration intervals, studies were categorized into daily and intermittent administration.
Forest plots with a random-effects model were used to explore the baseline characteristics and impact of each variable on the critical outcome. I2 statistics were used to assess the heterogeneity [15]. The pooled relative risks (RRs) were calculated for fractures or falls. The 95% confidence intervals (CIs) were calculated for each pooled value and are presented in square brackets throughout the manuscript.
The process of study screening, data extraction, and assessment of quality and risk of bias were performed by two independent reviewers (S.H.K. and H.N.J.). Quality assessment was performed using the Cochrane Collaboration tool for assessing risk of bias. This scale contains several items (two items on selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases). Each item was judged as ‘low risk,’ high risk,’ or ‘unclear risk’ of bias. Inconsistent ratings between the two investigators were reached through discussion [16]. Egger’s regression tests were performed to assess publication bias [17].
Analyses were performed for the outcomes of all osteoporotic fractures, hip fractures, and falls in the overall population and in subgroups according to the dose and interval. The effects of vitamin D supplementation on the outcomes were assessed separately according to the dose and interval categories: <800, 800 to 1,000, >1,000 IU/day for dose, and daily, intermittent administration for an interval. Sensitivity analyses were performed among studies without any restrictions in patient selection to reduce the heterogeneity of the results. All statistical analyses were performed using Stata version 16 (Stata statistical software: Release 16, StataCorp., College Station, TX, USA).
The initial search yielded 3,861 studies, which were narrowed down to 2,254 studies after duplicate removal. After screening, 1,970 studies were removed, and 284 articles were assessed using a full-text review. After removing 251 non-relevant studies, our systematic review included 32 studies (Fig. 1). The complete list and characteristics of the included studies are listed in Table 1 [7,10,18-47].
The 32 studies included 104,363 patients, with a median of 3,162 patients per study (range, 46 to 36,282). The studies were conducted in Europe (n=18), North America (n=10), Australasia (n=3), and Asia (n=1). Among them, 16 and 20 studies reported fractures and falls as outcomes, and 10 reported hip fractures. The median daily dose of cholecalciferol was 800 IU/day, and eight studies reported <800 IU/day, 15 studies reported 800 to 1,000 IU/day, and nine studies reported >1,000 IU/day. Regarding the interval, 26 studies reported daily administration, while six reported intermittent cholecalciferol administration. The median follow-up duration was 24 months (range, 9 to 120), and the median age was 72 years (range, 53 to 85). Most studies included women (32 [96.9%] studies), with 75% of participants (range, 15% to 100%) (Table 1).
Among the 32 studies, 16, 10, and 20 studies reported the risk of osteoporotic, hip fracture, and fall as outcomes. In terms of fractures, of 67,570 participants, 7,107 and 1,663 suffered osteoporotic and hip fractures, respectively. A meta-analysis of 16 studies revealed that vitamin D supplementation was not associated with a risk of osteoporotic fracture (pooled RR, 0.95; 95% CI, 0.86 to 1.04; I2=56.7%) (Fig. 2A). Although some studies published in the late 1990s reported preventive effects of vitamin D supplementation on the risk of fractures, most studies reported neutral effects, which were statistically insignificant overall. In a subgroup analysis, 10 studies reported hip fracture as an outcome. The pooled RR was 0.95 (95% CI, 0.81 to 1.10; I2=50.6%) (Fig. 2B). In terms of falls, 11,396 patients experienced falls during the follow-up. A meta-analysis of 21 studies showed that vitamin D supplementation was associated with a reduced risk of falls (pooled RR, 0.91; 95% CI, 0.85 to 0.98; I2=70.9%) (Fig. 2C). However, there was substantial evidence for heterogeneity in previous analyses, mainly due to different magnitudes of risk and follow-up duration across studies.
Subgroup analyses according to the daily dose of vitamin D supplementation were performed to improve the heterogeneity and determine the impact of dosage. The daily dose of 800 to 1,000 IU/day of vitamin D supplement was associated with a decreased fracture risk with a pooled RR of 0.87 (95% CI, 0.78 to 0.97; I2=23.5%), while vitamin D doses <800 and >1,000 IU/day were not associated with fracture risks (<800 IU/day: pooled RR, 0.97, 95% CI, 0.80 to 1.18; I2=61.7%; >1,000 IU/day: pooled RR, 1.14; 95% CI, 0.93 to 1.41; I2=44.1%) (Fig. 3A). In a subgroup analysis of hip fractures, vitamin D doses <800 and 800–1,000 IU/day were not significantly associated with the risk of hip fracture (<800 IU/day: pooled RR, 0.98; 95% CI, 0.81 to 1.19; I2=20.1%; 800–1,000 IU/day: pooled RR, 0.84; 95% CI, 0.64 to 1.10; I2=56.5%) (Fig. 3B).
Regarding falls, both <800 and 800–1,000 IU/day of vitamin D supplements showed a protective effect on the risk of falls (pooled RR, 0.89; 95% CI, 0.80 to 1.00; I2=0% and pooled RR, 0.81; 95% CI, 0.70 to 0.92; I 2=69.8%) (Fig. 3C). Besides, >1,000 IU/day of vitamin D supplements was not associated with the risk of falls.
Subgroup analyses were performed according to the intervals of vitamin D supplementation to determine the impact of the gap between administration. Subgroups were divided into two groups: daily and intermittent administration. The interval of administration was not significantly associated with the risk of osteoporotic fractures (daily: pooled RR, 0.94; 95% CI, 0.85 to 1.03; I2=49.0%; intermittent: pooled RR, 1.00; 95% CI, 0.64 to 1.58; I2=88.2%) (Fig. 4A). Intervals were not significantly associated with the risk of hip fractures also (Fig. 4B). However, daily administration of vitamin D supplementation was significantly associated with a reduced risk of falls (daily: pooled RR, 0.85; 95% CI, 0.76 to 0.95; I2=70.9%; intermittent: pooled RR, 1.01; 95% CI, 0.94 to 1.09; I2=52.1%) (Fig. 4C).
In a subgroup analysis regarding calcium supplementation, studies of calcium/vitamin D supplementation were associated with a significant risk reduction of falls, while risks of any osteoporotic fractures and hip fractures were not. (Fig. 5). Also, among studies with baseline vitamin D levels (n=23), vitamin D supplements were significantly related to reduced risks of falls in patients with vitamin D deficiency at their baseline.
Additionally, according to calcium supplementation, a subgroup analysis within the 800 to 1,000 IU/day group was done (Fig. 6). About fracture risk, studies with calcium/vitamin D supplementation showed significant risk reduction within 800-1,000 IU/day (pooled RR, 0.88; 95% CI, 0.78 to 1.00). On the other hand, the risk of fall was significantly reduced in both types of studies with both vitamin D only and calcium/vitamin D supplementation (vitamin D only: RR, 0.80; 95% CI, 0.65 to 0.97; calcium/vitamin D supplementation: RR, 0.81; 95% CI, 0.65 to 0.99). Also, in subgroup analysis according to institutionalized state, there was a significant reduction in the risk of falls in community-dwelling patients, while study numbers were insufficient to determine for institutionalized patients (Fig. 7).
In meta-regression analysis, baseline vitamin D level, age, percentage of women among study participants, and follow-up duration were insignificantly correlated with risk of any osteoporotic fracture (Supplemental Fig. S1), hip fracture (Supplemental Fig. S2), and fall (Supplemental Fig. S3).
Most studies at least partly met the quality standards of each area, while others did not. Among the 32 studies, five did not blind the participants and personnel, three did not adequately blind the outcome assessment, and five received funds from pharmaceuticals, which may cause reporting bias (Supplemental Fig. S4). Overall, there were concerns with quality in the four studies. Regarding publication bias, none of the studies showed significant publication bias in assessing risks of any osteoporotic, hip fractures, and falls (Supplemental Fig. S5).
The present meta-analysis included up-to-date randomized controlled trials (RCTs) with more than 100,000 patients to summarize the effect of vitamin D supplements on the risk of fractures and falls, according to different dosages and intervals. The analysis showed that vitamin D supplementation was associated with the reduced risk of fall, but not with fracture. However, vitamin D dose of 800 to 1,000 IU/day was associated with a 13% and 19% lower risk of fractures and falls, respectively. Also, daily administration of vitamin D was associated with decreased risk of falls, while intermittent administration was not. In patients with vitamin D deficiency, vitamin D supplementation showed a substantially reduced risk of falls. Correlations of participants’ age, sex, baseline vitamin D level, and follow-up duration with the risk of any osteoporotic, hip, and fall were all insignificant.
Consistent with previous meta-analyses [48], vitamin D supplementation showed a significant association with the risk of falls. It was also in agreement with a meta-analysis by Murad et al. [49] that vitamin D with calcium supplementation was related to the fall lowering effect. However, Bolland et al. [50] and US Preventive Services Task Force recommendation reported an insignificant association between vitamin D supplementation and fall [50-52]. The discrepancy among studies could be mainly due to the heterogeneity of study characteristics, such as various ways of vitamin D administration, insufficient number of participants, and short follow-up duration. From our analysis, including only studies with sufficient follow-up duration and event numbers, vitamin D3 could help reduce fall events. One of the feasible reasons for the effect of vitamin D on fall prevention is that vitamin D supplementation may help affect muscle strength, which could reduce body sway [18,53].
Regarding fracture outcome, it was also consistent with previous studies that vitamin D supplementation was not associated with the risk of fractures [6,48,54]. However, it has been suggested that some studies with negative results may not have enough events or follow-up duration to observe a meaningful difference [18,48]. In a recent meta-analysis, only a subgroup of follow-up duration >12 months showed a significant protective effect of vitamin D supplements on fracture risk [48], implying that the studies with enough follow-up duration can yield significant results. Also, there are some RCTs assessing very high annual doses of vitamin D that showed an increased risk of fractures and falls in participants who received vitamin D [19,55]. Various designs and administration methods of vitamin D may confuse and attenuate the final result, even though we excluded studies with short duration and small event numbers [6,48,54]. In addition, fragility fractures are complex events that many factors contribute simultaneously, such as physical activity, balancing ability, and especially, bone density [56,57]. Therefore, although efforts were made to include selected studies, vitamin D supplementation alone may not result in a significant difference in fracture outcome.
To overcome the pitfalls of heterogeneity and find the best way to replace vitamin D, subgroup analyses according to different dosages were performed. Vitamin D supplements of 800 to 1,000 IU/day reduced the risk of osteoporotic fractures and falls with low heterogeneity. These results are consistent with previous findings that a moderate dose of vitamin D supplements may help reduce fracture and fall risk [20-22,58]. It is notable that many previous studies involving vitamin D supplements of 800 to 1,000 IU/day are based on the institutionalized population [20,21]. However, only a few studies were based on institutionalized patients in this analysis [20,21], mainly because most previous studies had a short follow-up duration and a small number of participants, making the main population of the study community-dwelling. Therefore, the result implies that community-dwelling patients may also benefit from taking vitamin D. Interestingly, subgroup analysis of the calculated daily dose >1,000 IU showed a trend of increased risk of fractures and falls, although it was insignificant. A recent study by Bolland et al. [50] showed similar results, along with other meta-analyses [59,60], that intermittent vitamin D supplements raised fall risks. Although the reasons are not clear, intermittent supplements are usually given in high doses that are suspected to be the cause of increased fractures and falls. Some studies suggested the U-shaped association between vitamin D and risk of fractures and falls, which could be mediated via the vitamin D receptor in the central nervous system [59,60]. Also, as the half-life of 25-hydroxyvitamin D (25(OH)D) is approximately 15 days, monthly or yearly intervals are likely to cause fluctuations that may lead to toxic levels of 25(OH)D in the blood [19,22,60]. To summarize, a moderate dose of 800 to 1,000 IU and daily administration of vitamin D can be beneficial in preventing osteoporotic fractures and falls in the general population.
In addition, patients who had vitamin D deficiency at their baseline could benefit from vitamin D supplements, which showed a 22% reduction in the risk of fall in our data. The results could be partly explained by previous studies that vitamin D supplementation improved functional outcomes, such as lower limb strength and balance in elderly patients with vitamin D deficiency [61]. Besides, in association analysis, vitamin D level, calcium supplementation, age, the proportion of women, and follow-up duration were not associated with the risk of fractures or falls. However, it could be due to the high heterogeneity of included studies in the association analysis. Especially, baseline vitamin D levels were presented in only nine studies, which may mislead the result of linear association, unlike subgroup analysis. More studies are needed to conclude that age and additional calcium supplementation affect the risk of fractures and falls during vitamin D supplementation.
Overall, the key to the success of trials of vitamin D supplements could be the study design to achieve targeted 25(OH)D levels in the selected population. As there is a strong correlation between baseline vitamin D level and bone mineral density [4,5], muscle mass, and function [2], the inconsistent results in the RCTs can be largely influenced by subject selection and vitamin D concentration at the baseline. Also, 25(OH)D levels reached with the fixed doses can vary greatly. Further trials with a design targeting optimal levels with flexible dosages in a selected population are required.
This study has several strengths. As there was a need for integrated updated meta-analysis due to recently published RCTs [7,10,25], our analysis has its strength in integrating the recent results until March 2021. Also, the study confirmed previous knowledge and revealed some novel findings. We found a significantly decreased risk of fractures and falls with vitamin D supplements of 800 to 1,000 IU/day. Also, as intervals of vitamin D supplementation were separately analyzed, the results from subgroup analyses may help determine the dosage and intervals of vitamin D supplementation in clinical practice. The updated meta-analysis differs from previous meta-analyses [50,54] in that it excluded RCTs with short follow-up durations (i.e., <6 months) or those including few fracture events (i.e., <10 events) to minimize the risks of bias. Also, studies regarding ergocalciferol were not included in the analysis to reduce the heterogeneity of studies.
This study also has some limitations. First, only a few studies selected for the review were conducted on institutionalized patients due to the limitation of follow-up duration and number of events. However, it may help reduce the heterogeneity of analysis that studies focusing on specific clinical conditions to evaluate the treatment of vitamin D deficiency to improve the disease or symptoms were excluded from the analysis. Most of the studies were mainly on community-dwelling populations. Second, residual heterogeneity was observed in some subgroup analyses. The residual extent of heterogeneity may be partially explained by differences in age distribution, underlying diseases, or nutritional status among the studies.
Our meta-analyses summarized the effects of vitamin D supplements at different dosages and intervals on the risk of fractures and falls. Among the various administration methods, only a daily dose of 800 to 1,000 IU may reduce the risk of osteoporotic fractures and falls. We also identified that a daily interval of vitamin D supplementation could help reduce the risk of falls. Also, vitamin D deficient patients were more likely to benefit from vitamin D supplementation by reducing the risk of falls. To summarize, consistent with previous recommendations, a daily vitamin D dose of 800 to 1,000 IU was the most probable way to reduce the fracture and fall risk. As it is not possible that one regimen suits all, further studies with various regimens targeting vitamin D levels are required to elucidate the benefits of vitamin D supplements.
Supplementary Information
Supplemental Fig. S1.
Meta-regression plot of risk of osteoporotic fractures with (A) baseline vitamin D level, (B) age, (C) percentage of women, and (D) follow-up duration. CI, confidence interval.
Supplemental Fig. S2.
Meta-regression plot of risk of hip fractures with (A) baseline vitamin D level, (B) age, (C) percentage of women, and (D) follow-up duration. CI, confidence interval.
Supplemental Fig. S3.
Meta-regression plot of risk of fall with (A) baseline vitamin D level, (B) age, (C) percentage of women, and (D) follow-up duration. CI, confidence interval.
Supplemental Fig. S5.
Funnel plots of the risks of (A) any osteoporotic, (B) hip fracture, and (C) fall.
REFERENCES
2. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003; 88:5766–72.

3. Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality: a review of recent evidence. Autoimmun Rev. 2013; 12:976–89.
4. Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009; 94:1244–50.

5. Cauley JA, Greendale GA, Ruppert K, Lian Y, Randolph JF Jr, Lo JC, et al. Serum 25 hydroxyvitamin D, bone mineral density and fracture risk across the menopause. J Clin Endocrinol Metab. 2015; 100:2046–54.

6. Kahwati LC, LeBlanc E, Weber RP, Giger K, Clark R, Suvada K, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021; 325:1443–63.
7. Appel LJ, Michos ED, Mitchell CM, Blackford AL, Sternberg AL, Miller ER 3rd, et al. The effects of four doses of vitamin D supplements on falls in older adults: a responseadaptive, randomized clinical trial. Ann Intern Med. 2021; 174:145–56.

8. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96:53–8.

9. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011; 96:1911–30.

10. LeBoff MS, Murata EM, Cook NR, Cawthon P, Chou SH, Kotler G, et al. VITamin D and OmegA-3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab. 2020; 105:2929–38.

11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016; 75:40–6.
12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–12.

14. Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev. 2021; 10:39.

16. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006; 144:427–37.

17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.

18. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000; 15:1113–8.

19. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010; 303:1815–22.

20. Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ. 1994; 308:1081–2.

21. Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk. The Decalyos II study. Osteoporos Int. 2002; 13:257–64.

22. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003; 326:469.

23. Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015; 373:1519–30.

24. Salovaara K, Tuppurainen M, Karkkainen M, Rikkonen T, Sandini L, Sirola J, et al. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a populationbased 3-year randomized, controlled trial. The OSTPREFPS. J Bone Miner Res. 2010; 25:1487–95.

25. Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva J, Blauth M, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020; 324:1855–68.

26. Khaw KT, Stewart AW, Waayer D, Lawes C, Toop L, Camargo CA Jr, et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017; 5:438–47.

27. Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc. 2017; 65:323–31.

28. Hin H, Tomson J, Newman C, Kurien R, Lay M, Cox J, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int. 2017; 28:841–51.

29. Imaoka M, Higuchi Y, Todo E, Kitagwa T, Ueda T. Low-frequency exercise and vitamin d supplementation reduce falls among institutionalized frail elderly. Int J Gerontol. 2016; 10:202–6.

30. Cangussu LM, Nahas-Neto J, Orsatti CL, Poloni PF, Schmitt EB, Almeida-Filho B, et al. Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause. 2016; 23:267–74.
31. Uusi-Rasi K, Patil R, Karinkanta S, Kannus P, Tokola K, Lamberg-Allardt C, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. 2015; 175:703–11.

32. Hansen KE, Johnson RE, Chambers KR, Johnson MG, Lemon CC, Vo TN, et al. Treatment of vitamin D insufficiency in postmenopausal women: a randomized clinical trial. JAMA Intern Med. 2015; 175:1612–21.

33. Wood AD, Secombes KR, Thies F, Aucott LS, Black AJ, Reid DM, et al. A parallel group double-blind RCT of vitamin D3 assessing physical function: is the biochemical response to treatment affected by overweight and obesity? Osteoporos Int. 2014; 25:305–15.

34. Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013; 24:567–80.

35. Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013; 173:1672–9.
36. Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. 2012; 27:170–6.

37. Karkkainen M, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65-71 years: a 3-year randomized population-based trial (OSTPRE-FPS). Osteoporos Int. 2010; 21:2047–55.
38. Pfeifer M, Begerow B, Minne HW, Suppan K, FahrleitnerPammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009; 20:315–22.

39. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006; 166:424–30.

40. Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005; 330:1003.

41. Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005; 365:1621–8.

42. Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents severe falls in elderly community-dwelling women: a pragmatic population-based 3-year intervention study. Aging Clin Exp Res. 2005; 17:125–32.

43. Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res. 2002; 17:709–15.

44. Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, et al. Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab. 2000; 85:3011–9.

45. Komulainen MH, Kroger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. HRT and Vit D in prevention of non-vertebral fractures in postmenopausal women: a 5 year randomized trial. Maturitas. 1998; 31:45–54.

46. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997; 337:670–6.

47. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996; 124:400–6.

48. Thanapluetiwong S, Chewcharat A, Takkavatakarn K, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P. Vitamin D supplement on prevention of fall and fracture: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020; 99:e21506.
49. Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011; 96:2997–3006.
50. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018; 6:847–58.

51. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018; 319:1696–704.
52. US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Cabana M, Caughey AB, et al. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021; 325:1436–42.
53. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014; 99:4336–45.

54. Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open. 2019; 2:e1917789.
55. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women: a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007; 46:1852–7.

56. Martin FC. Falls risk factors: assessment and management to prevent falls and fractures. Can J Aging. 2011; 30:33–44.

57. Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc. 2000; 48:883–93.

58. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005; 293:2257–64.
59. Chua GT, Wong RY. Association between vitamin D dosing regimen and fall prevention in long-term care seniors. Can Geriatr J. 2011; 14:93–9.

Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) diagram of study selection.
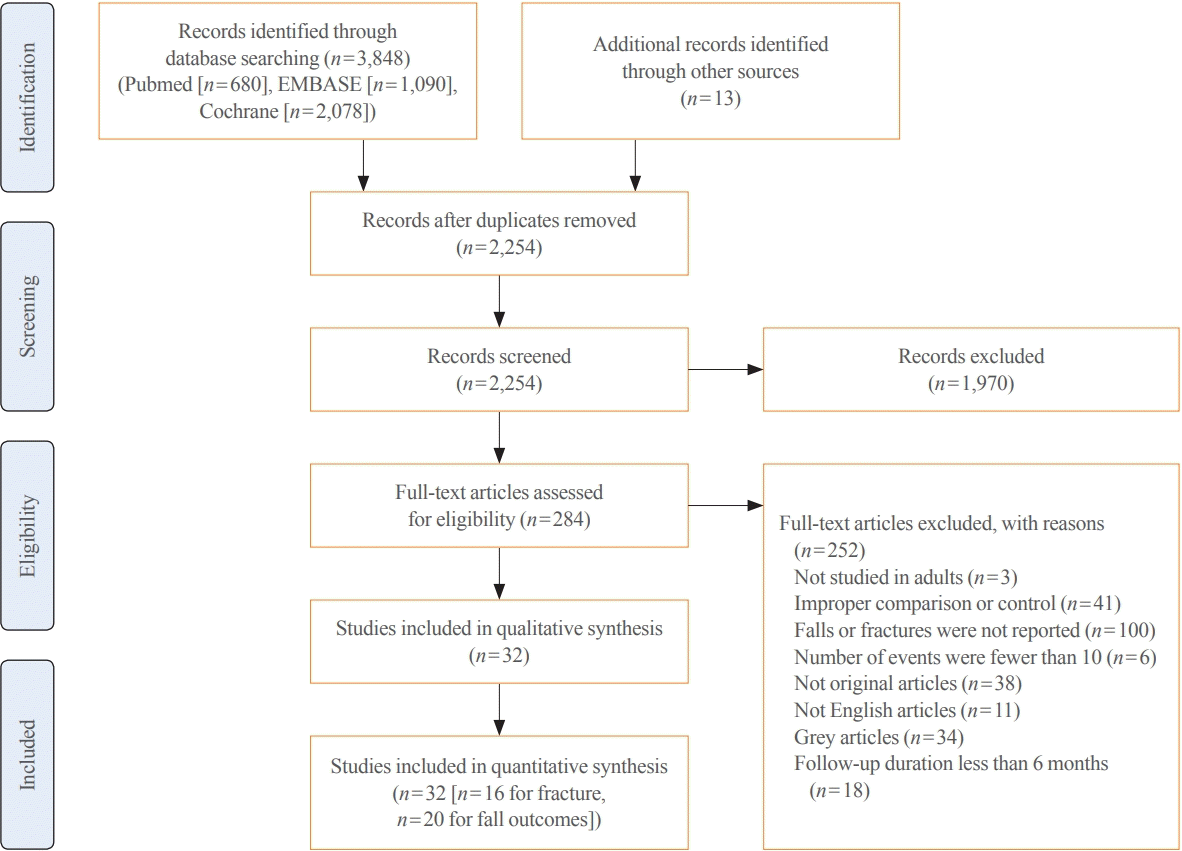
Fig. 2.
Impacts of vitamin D supplements on the risks of (A) any osteoporotic, (B) hip fracture, and (C) fall. CI, confidence interval.
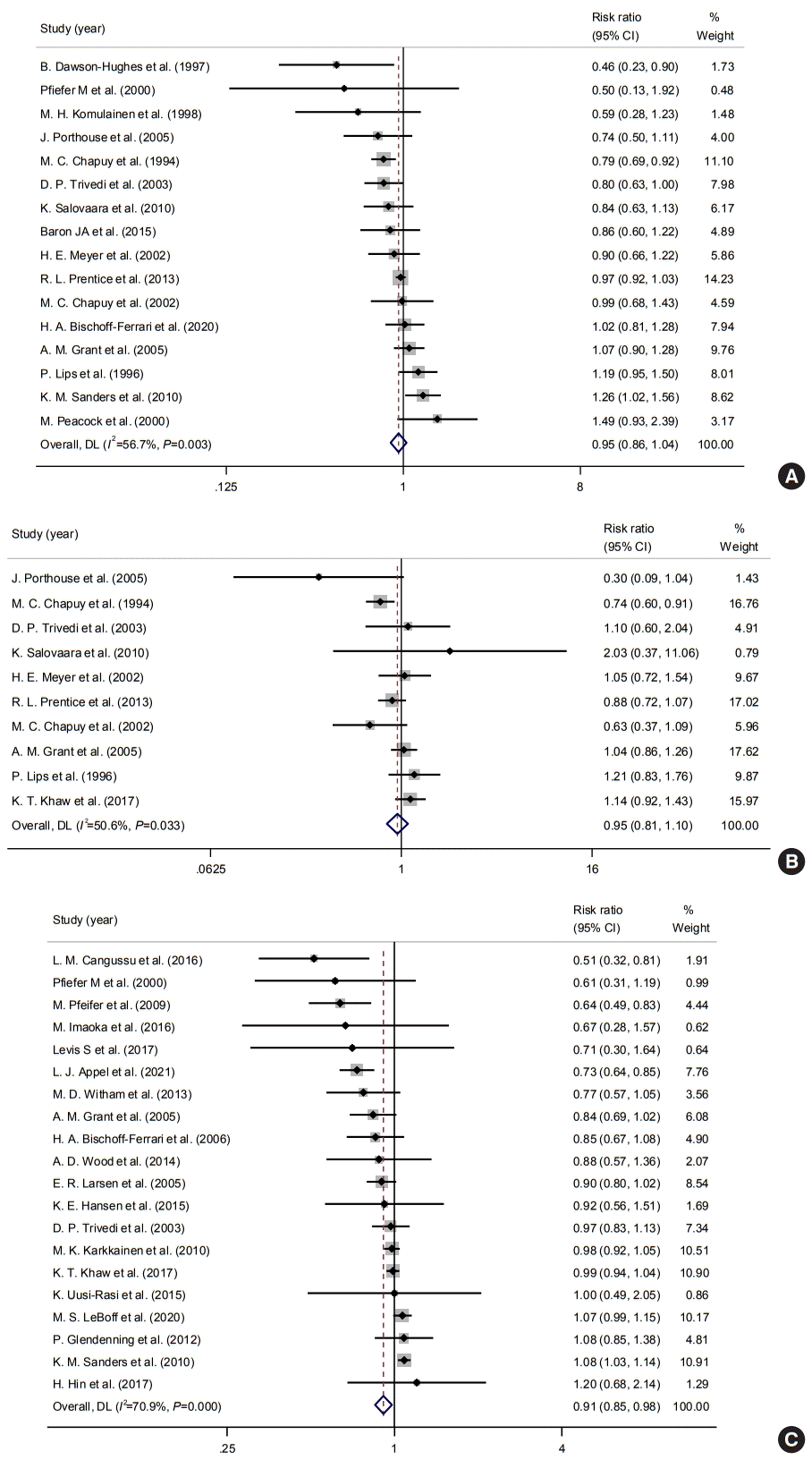
Fig. 3.
Impacts of vitamin D supplements on the risks of (A) any osteoporotic, (B) hip fracture, and (C) fall according to daily dosages. CI, confidence interval.
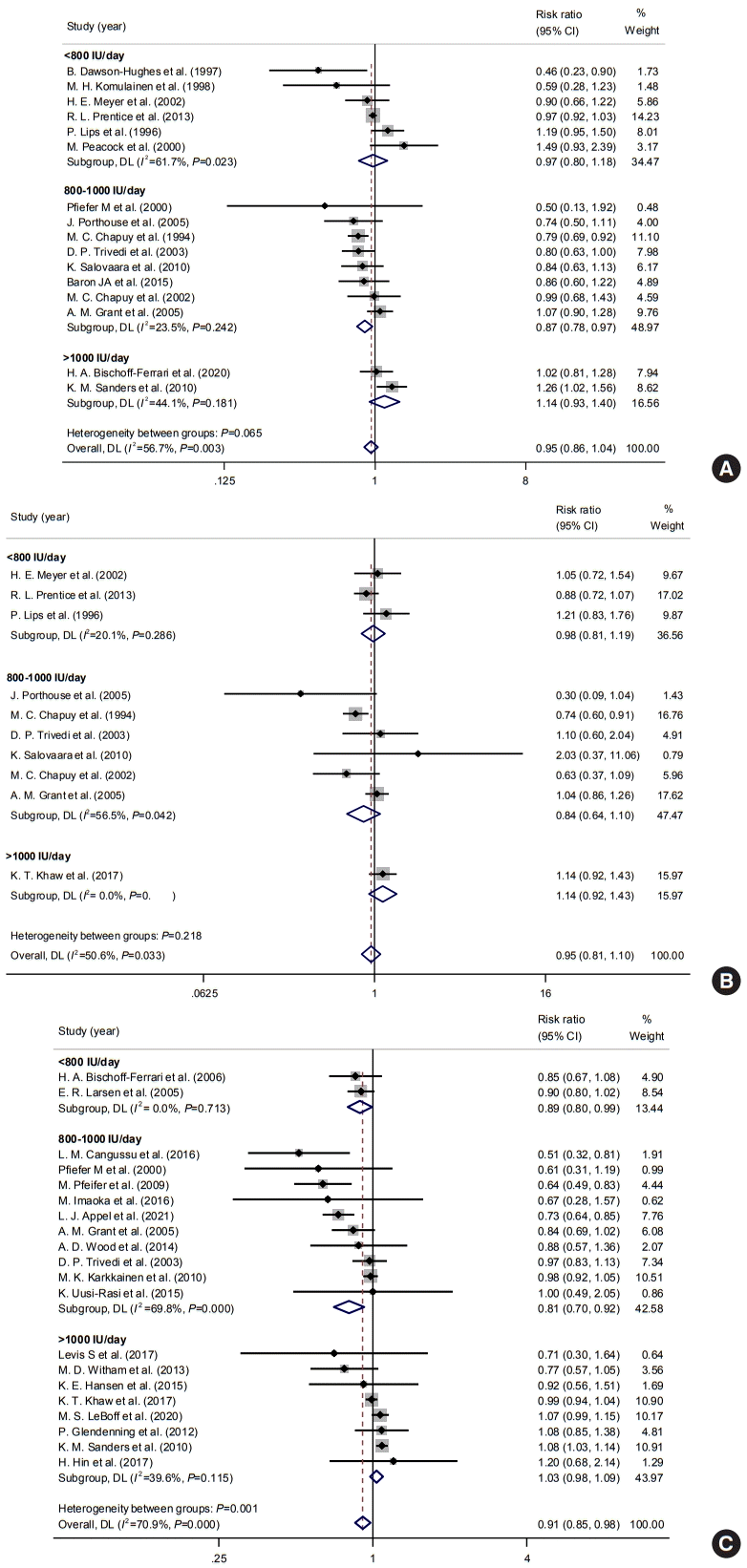
Fig. 4.
Impacts of vitamin D supplements on the risks of (A) any osteoporotic, (B) hip fracture, and (C) fall according to intervals. CI, confidence interval.
Fig. 5.
Impacts of vitamin D supplements on the risks of any osteoporotic, hip fracture, and fall according to combined calcium supplementation and baseline vitamin D level. CI, confidence interval.
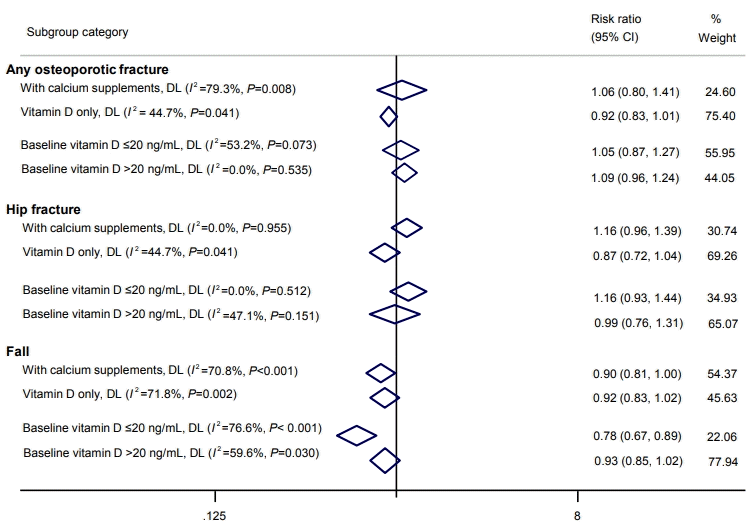
Fig. 6.
Impacts of 800 to 1,000 IU/day of vitamin D supplements on the risks of (A) osteoporotic fracture and (B) fall according to combined calcium supplementation. CI, confidence interval.
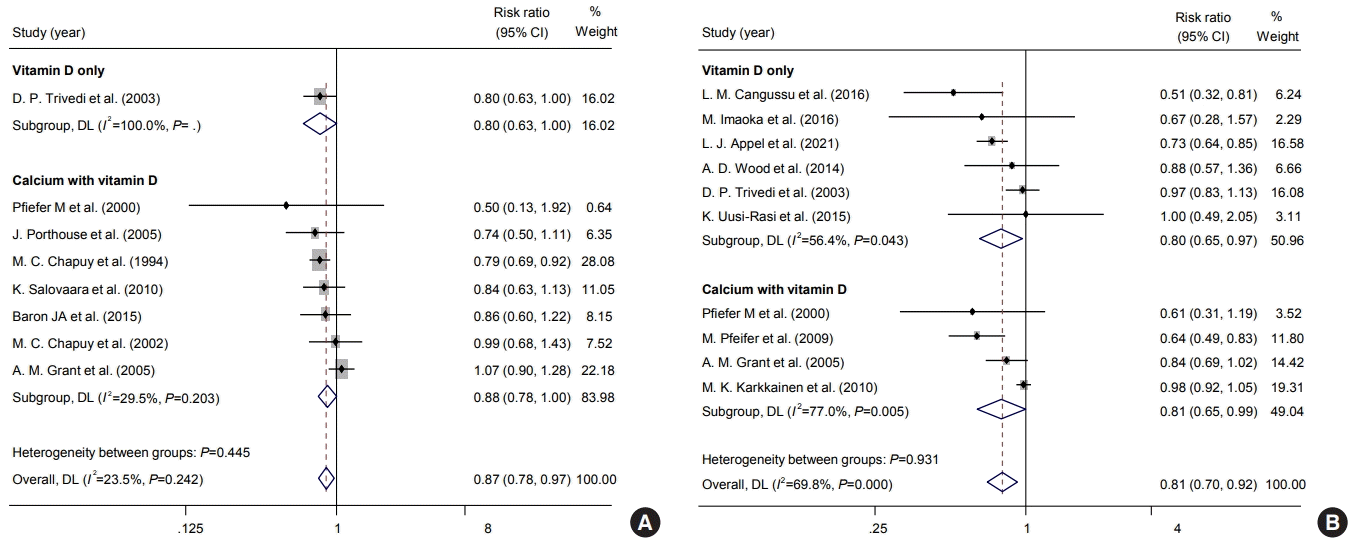
Fig. 7.
Impacts of vitamin D supplements on the risks of (A) osteoporotic fracture and (B) fall according to institutionalized and communitydwelling populations. CI, confidence interval.
Table 1.
Study Characteristics of Randomized Controlled Trials Reporting Effects of Vitamin D Treatment on Risk of Fractures and Falls
| Source | Location | No. of participants | Interventions | FU duration, mo | Age, yr | Women, % | Outcomes reported | No. of events |
|---|---|---|---|---|---|---|---|---|
| Appel et al. (2021) [7] | US | 688 | 1,000, 2,000, or 4,000 IU of vitamin D3 per day for dose-finding stage | 24 | 77.2 | 43.6 | Fall | 365 |
| 1,000 IU/day for confirmatory stage | ||||||||
| Bischoff-Ferrari et al. (2020) [25] | Switzerland | 2,157 | 2×2×2 factorial design | 36 | 74.9 | 61.7 | Fracture | 256 |
| 2,000 IU/day of vitamin D3 and calcium supplement 500 mg/day, 1 g/day of omega-3s, and a strength-training exercise program; vitamin D3 and omega-3s; vitamin D3 and exercise; vitamin D3 alone; omega-3s and exercise; omega-3s alone; exercise alone; or placebo | ||||||||
| LeBoff et al. (2020) [10] | US | 25,871 | 2×2 factorial design | 63.6 | 67.1 | 51 | Fall | 2,329 |
| 2,000 IU/day of vitamin D3 and calcium supplements and/or omega-3s 1 g/day or respective placebos | ||||||||
| Khaw et al. (2017) [26] | New Zealand | 5,110 | Initial oral dose of 200,000 IU vitamin D3 followed by monthly 100,000 IU vitamin D3 or equivalent placebo | 41 | 65.9 | 42 | Fracture | 2,638 |
| Fall | ||||||||
| Levis et al. (2017) [27] | US | 130 | 4,000 IU per day of vitamin D3 or placebo | 9 | 72.4 | 0 | Fall | 19 |
| Hin et al. (2017) [28] | UK | 305 | 4,000, 2,000 IU per day of vitamin D3 or placebo | 12 | 71 | 49 | Fall | 48 |
| Imaoka et al. (2016) [29] | Japan | 91 | 900 IU/day of vitamin D or placebo | 9 | 82 | 75.8 | Fall | 15 |
| Cangussu et al. (2016) [30] | Brazil | 160 | 1,000 IU/day of vitamin D3 or placebo | 12 | 59 | 100 | Fall | 56 |
| Baron et al. (2015) [23] | US | 2,259 | Partial 2×2 factorial design | 60 | 58 | 15 | Fracture | 119 |
| 1,000 IU per day of vitamin D3, 1,200 mg/ day of calcium carbonate, both, or neither | ||||||||
| Uusi-Rasi et al. (2015) [31] | Finland | 409 | Placebo without exercise, vitamin D3 (800 IU/day) without exercise, placebo and exercise, and vitamin D3 (800 IU/day) and exercise | 24 | 74 | 100 | Fall | 26 |
| Hansen et al. (2015) [32] | US | 230 | Daily white and twice monthly yellow placebo, daily 800 IU vitamin D3 and twice monthly yellow placebo, and daily white placebo and twice monthly 50,000 IU vitamin D3 (n=79) | 12 | 61 | 100 | Fall | 45 |
| Wood et al. (2014) [33] | UK | 305 | 400 or 1,000 IU per daily of vitamin D3 or placebo | 12 | 63.8 | 100 | Fall | 58 |
| Prentice et al. (2013) [34] | US | 36,282 | 1,000 mg elemental calcium carbonate plus 400 IU of vitamin D3 daily or placebo | 86 | 65 | 100 | Fracture | 4,260 |
| Witham et al. (2013) [35] | UK | 159 | 100,000 IU of oral cholecalciferol every 3 months or placebo | 12 | 77 | 50 | Fall | 82 |
| Glendenning et al. (2012) [36] | Australia | 686 | 150,000 IU every 3 months of oral cholecalciferol or placebo | 9 | 76 | 100 | Fall | 191 |
| Salovaara et al. (2010) [24] | Finland | 3,195 | 800 IU of cholecalciferol and 1,000 mg of calcium carbonate or control without placebo | 36 | 67 | 100 | Fracture | 172 |
| Sanders et al. (2010) [19] | Australia | 137 | 500,000 IU of oral cholecalciferol annually or placebo | 60 | 76 | 100 | Fracture | 306 |
| Fall | 1,606 | |||||||
| Karkkainen et al. (2010) [37] | Finland | 3,139 | 800 IU of cholecalciferol and 1,000 mg of calcium carbonate or control without placebo | 36 | 67 | 100 | Fall | 1,645 |
| Pfeifer et al. (2009) [38] | Germany | 242 | 1,000 mg of calcium or 1,000 mg of calcium plus 800 IU of vitamin D3 per day | 12 | 77 | 100 | Fall | 124 |
| Bischoff-Ferrari et al. (2006) [39] | US | 445 | 700 IU of vitamin D3 plus 500 mg of calcium citrate per day or placebo | 36 | 71 | 55 | Fall | 170 |
| Porthouse et al. (2005) [40] | UK | 3,314 | Daily oral supplementation using 1,000 mg calcium with 800 IU cholecaliferol or control without placebo | 25 | 77 | 100 | Fracture | 103 |
| Grant et al. (2005) [41] | UK | 5,292 | 800 IU daily oral vitamin D3, 1,000 mg calcium, oral vitamin D3 plus calcium (1,000 mg per day), or placebo | 24 | 77 | 100 | Fracture | 408 |
| Fall | 415 | |||||||
| Larsen et al. (2005) [42] | Denmark | 9,605 | 1,000 mg of calcium carbonate and 400 IU of vitamin D3 daily or control | 42 | 74 | 60.1 | Fall | 913 |
| Trivedi et al. (2003) [22] | UK | 2,686 | 100,000 IU oral vitamin D3 or matching placebo every 4 months | 60 | 74 | 24.1 | Fracture | 268 |
| Fall | 515 | |||||||
| Chapuy et al. (2002) [21] | France | 583 | 800 IU of vitamin D3 plus 1,200 mg calcium carbonate or placebo | 24 | 85 | 100 | Fracture | 105 |
| Fall | ||||||||
| Meyer et al. (2002) [43] | Norway | 1,144 | Ordinary cod liver oil (400 IU of vitamin D3) or cod liver oil where vitamin D was removed | 24 | 85 | 75 | Fracture | 145 |
| Pfeifer et al. (2000) [18] | Germany | 148 | 1,200 mg of calcium carbonate or 1,200 mg of elemental calcium and 800 IU of vitamin D3 | 12 | 74 | 100 | Fracture | 9 |
| Fall | 35 | |||||||
| Peacock et al. (2000) [44] | US | 438 | 750 mg calcium citrate plus 600 IU of vitamin D3 or placebo | 48 | 75 | 71.7 | Fracture | 56 |
| Komulainen et al. (1998) [45] | Finland | 464 | 300 IU/day of vitamin D3 or placebo | 120 | 53 | 100 | Fracture | 27 |
| Dawson-Hughes et al. (1997) [46] | US | 389 | 500 mg of calcium plus 700 IU of vitamin D3 per day or placebo | 36 | 71 | 54.7 | Fracture | 37 |
| Lips et al. (1996) [47] | Belgium | 2,578 | Vitamin D3, 400 IU in one tablet daily, or placebo | 42 | 80 | 74.3 | Fracture | 267 |
| Chapuy et al. (1994) [20] | France | 3,270 | 1–2 g calcium daily in the form of tricalcium phosphate, together with 800 IU cholecalciferol or placebo | 18 | 72 | 100 | Fracture | 563 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download