1. Pondel M. Calcitonin and calcitonin receptors: bone and beyond. Int J Exp Pathol. 2000; 81:405–22.

2. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003; 13:3–126.
3. Engelbach M, Gorges R, Forst T, Pfutzner A, Dawood R, Heerdt S, et al. Improved diagnostic methods in the follow-up of medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin Endocrinol Metab. 2000; 85:1890–4.

4. Guilloteau D, Perdrisot R, Calmettes C, Baulieu JL, Lecomte P, Kaphan G, et al. Diagnosis of medullary carcinoma of the thyroid (MCT) by calcitonin assay using monoclonal antibodies: criteria for the pentagastrin stimulation test in hereditary MCT. J Clin Endocrinol Metab. 1990; 71:1064–7.

5. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015; 25:567–610.

6. Ball DW. Medullary thyroid cancer: monitoring and therapy. Endocrinol Metab Clin North Am. 2007; 36:823–37.

7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

8. Hodak SP, Burman KD. The calcitonin conundrum: is it time for routine measurement of serum calcitonin in patients with thyroid nodules? J Clin Endocrinol Metab. 2004; 89:511–4.
9. Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004; 89:163–8.

10. Hahm JR, Lee MS, Min YK, Lee MK, Kim KW, Nam SJ, et al. Routine measurement of serum calcitonin is useful for early detection of medullary thyroid carcinoma in patients with nodular thyroid diseases. Thyroid. 2001; 11:73–80.

11. Niccoli P, Wion-Barbot N, Caron P, Henry JF, de Micco C, Saint Andre JP, et al. Interest of routine measurement of serum calcitonin: study in a large series of thyroidectomized patients. The French Medullary Study Group. J Clin Endocrinol Metab. 1997; 82:338–41.
12. Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007; 92:450–5.

13. Chambon G, Alovisetti C, Idoux-Louche C, Reynaud C, Rodier M, Guedj AM, et al. The use of preoperative routine measurement of basal serum thyrocalcitonin in candidates for thyroidectomy due to nodular thyroid disorders: results from 2733 consecutive patients. J Clin Endocrinol Metab. 2011; 96:75–81.

14. Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016; 9:59–126.

15. Toledo SP, Lourenco DM Jr, Santos MA, Tavares MR, Toledo RA, Correia-Deur JE. Hypercalcitoninemia is not pathognomonic of medullary thyroid carcinoma. Clinics (Sao Paulo). 2009; 64:699–706.

16. Niccoli P, Conte-Devolx B, Lejeune PJ, Carayon P, Henry JF, Roux F, et al. Hypercalcitoninemia in conditions other than medullary cancers of the thyroid. Ann Endocrinol (Paris). 1996; 57:15–21.
17. Colombo C, Verga U, Mian C, Ferrero S, Perrino M, Vicentini L, et al. Comparison of calcium and pentagastrin tests for the diagnosis and follow-up of medullary thyroid cancer. J Clin Endocrinol Metab. 2012; 97:905–13.

18. Karanikas G, Moameni A, Poetzi C, Zettinig G, Kaserer K, Bieglmayer C, et al. Frequency and relevance of elevated calcitonin levels in patients with neoplastic and nonneoplastic thyroid disease and in healthy subjects. J Clin Endocrinol Metab. 2004; 89:515–9.

19. Kwon H, Kim WG, Choi YM, Jang EK, Jeon MJ, Song DE, et al. A cut-off value of basal serum calcitonin for detecting macroscopic medullary thyroid carcinoma. Clin Endocrinol (Oxf). 2015; 82:598–603.

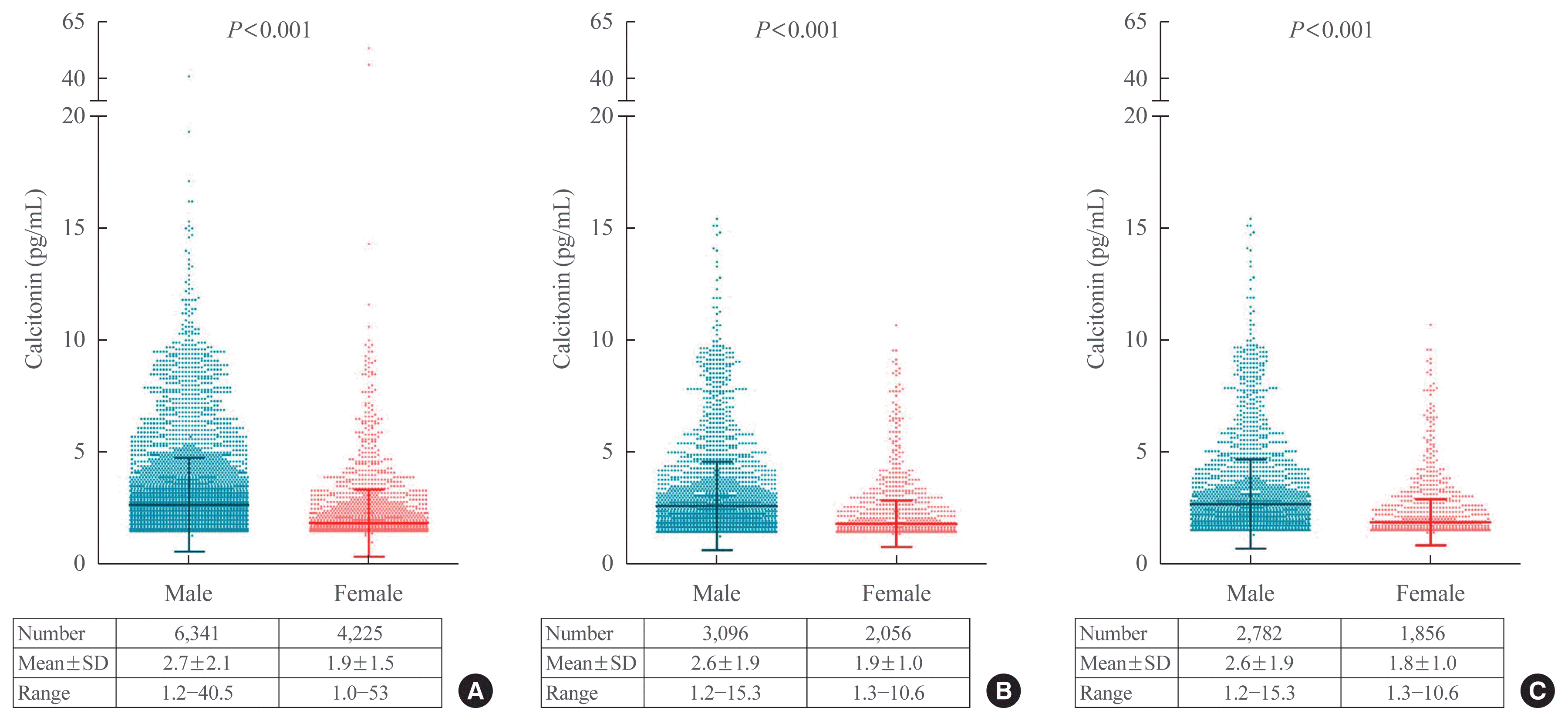
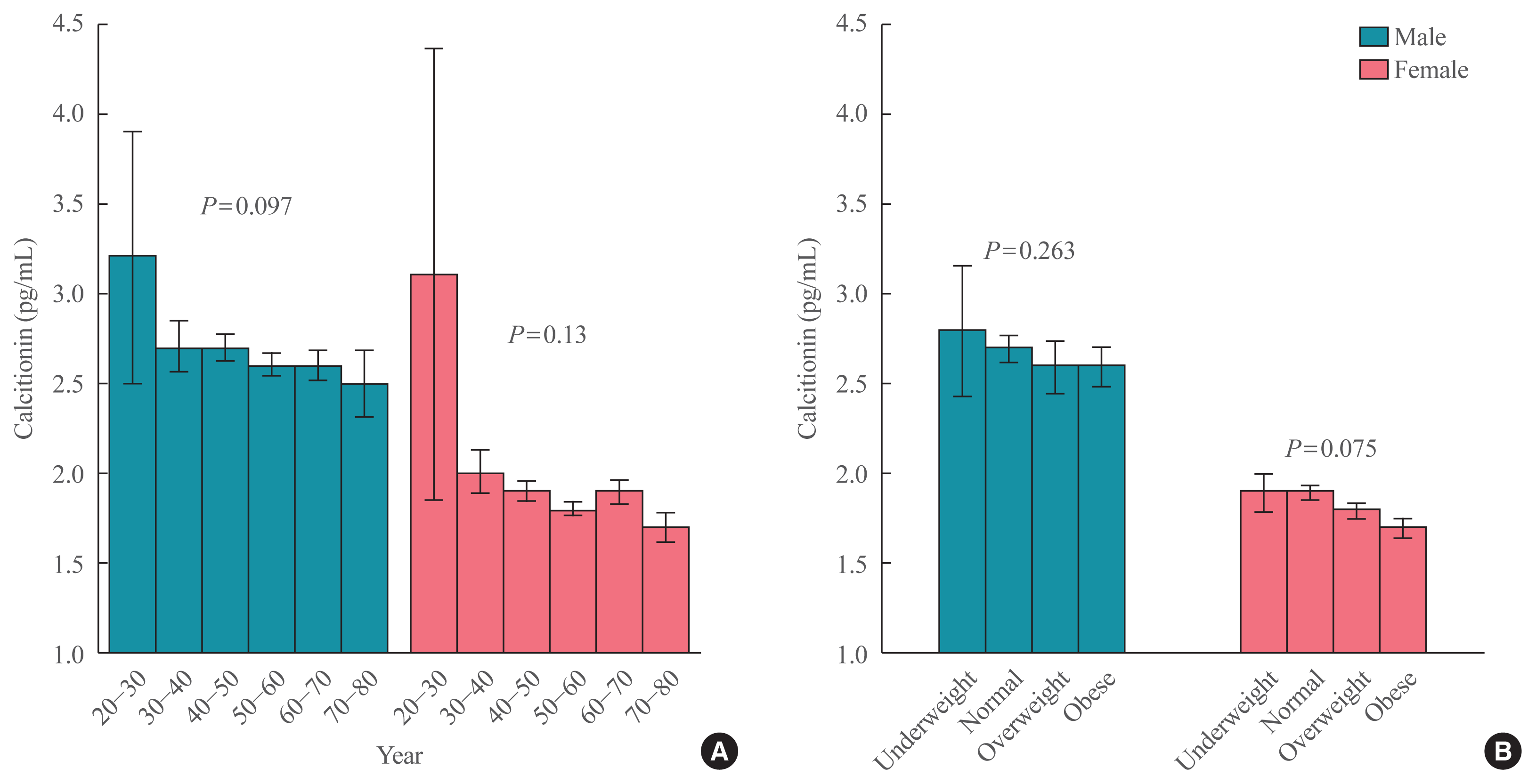
20. d’Herbomez M, Caron P, Bauters C, Do Cao C, Schlienger JL, Sapin R, et al. Reference range of serum calcitonin levels in humans: influence of calcitonin assays, sex, age, and cigarette smoking. Eur J Endocrinol. 2007; 157:749–55.

21. Basuyau JP, Mallet E, Leroy M, Brunelle P. Reference intervals for serum calcitonin in men, women, and children. Clin Chem. 2004; 50:1828–30.

22. Verga U, Morpurgo PS, Vaghi I, Radetti G, Beck-Peccoz P. Normal range of calcitonin in children measured by a chemiluminescent two-site immunometric assay. Horm Res. 2006; 66:17–20.

23. Baylin SB, Bailey AL, Hsu TH, Foster GV. Degradation of human calcitonin in human plasmas. Metabolism. 1977; 26:1345–54.
24. Elisei R. Routine serum calcitonin measurement in the evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008; 22:941–53.

25. Niccoli P, Brunet P, Roubicek C, Roux F, Baudin E, Lejeune PJ, et al. Abnormal calcitonin basal levels and pentagastrin response in patients with chronic renal failure on maintenance hemodialysis. Eur J Endocrinol. 1995; 132:75–81.

26. World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000. p. 55.
27. Guyetant S, Rousselet MC, Durigon M, Chappard D, Franc B, Guerin O, et al. Sex-related C cell hyperplasia in the normal human thyroid: a quantitative autopsy study. J Clin Endocrinol Metab. 1997; 82:42–7.

28. Garcia-Ameijeiras A, De La Torre W, Rodriguez-Espinosa J, Perez-Perez A, De Leiva A. Does testosterone influence the post-stimulatory levels of calcitonin in normal men? Clin Endocrinol (Oxf). 1987; 27:545–52.

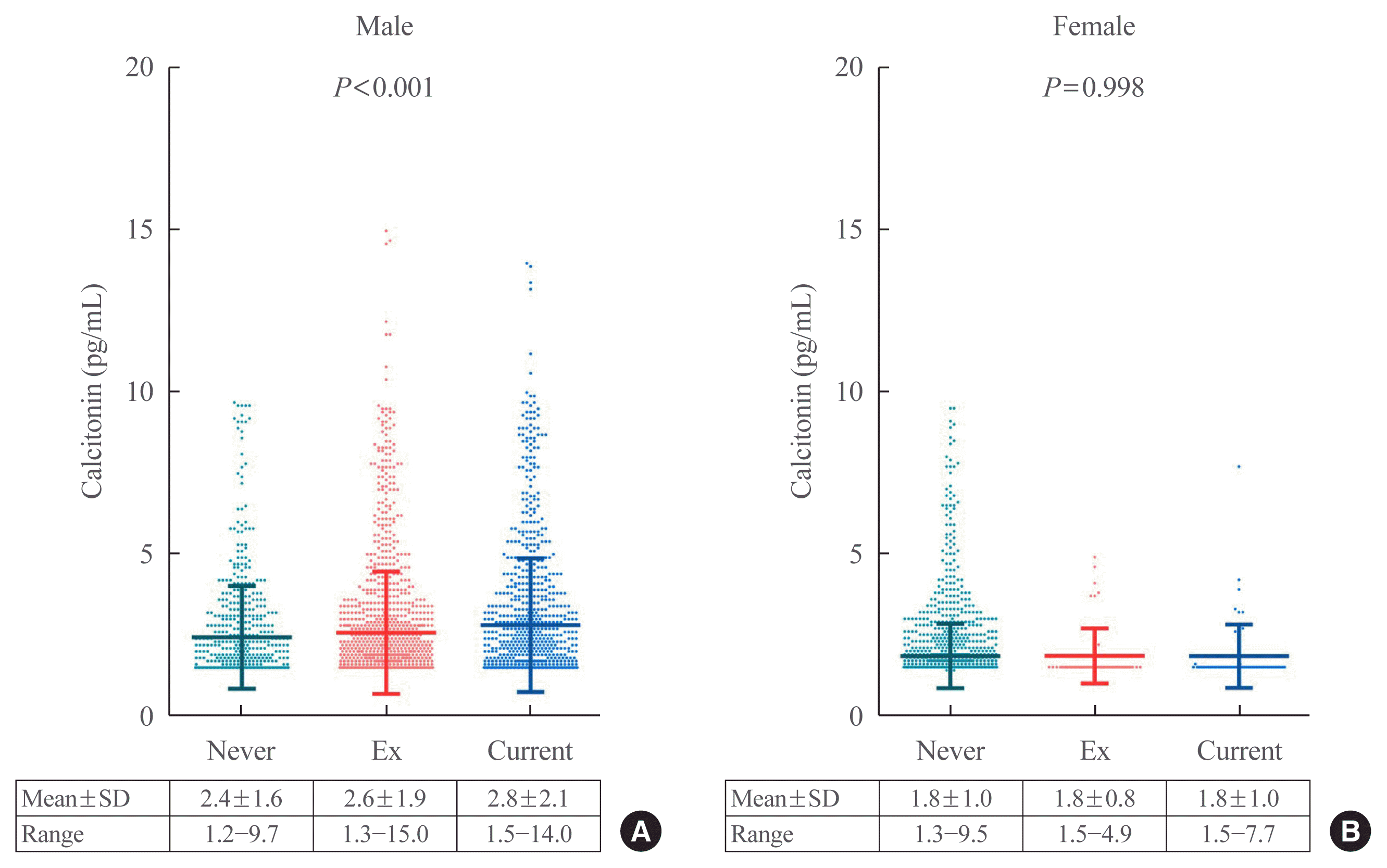
29. Vestergaard P. Smoking and thyroid disorders: a meta-analysis. Eur J Endocrinol. 2002; 146:153–61.
30. Schlienger JL, Grunenberger F, Vinzio S, Goichot B. Smoking and the thyroid. Ann Endocrinol (Paris). 2003; 64:309–15.
31. Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005; 152:491–9.

32. Tabassian AR, Nylen ES, Giron AE, Snider RH, Cassidy MM, Becker KL. Evidence for cigarette smoke-induced calcitonin secretion from lungs of man and hamster. Life Sci. 1988; 42:2323–9.

33. Vierhapper H, Raber W, Bieglmayer C, Kaserer K, Weinhausl A, Niederle B. Routine measurement of plasma calcitonin in nodular thyroid diseases. J Clin Endocrinol Metab. 1997; 82:1589–93.

34. Fereira-Valbuena H, Fernandez de Arguello E, Campos G, Ryder E, Avellaneda A. Serum concentration of calcium and calcitonin in hyperthyroidism caused by Graves’ disease. Invest Clin. 1991; 32:109–14.




 PDF
PDF Citation
Citation Print
Print



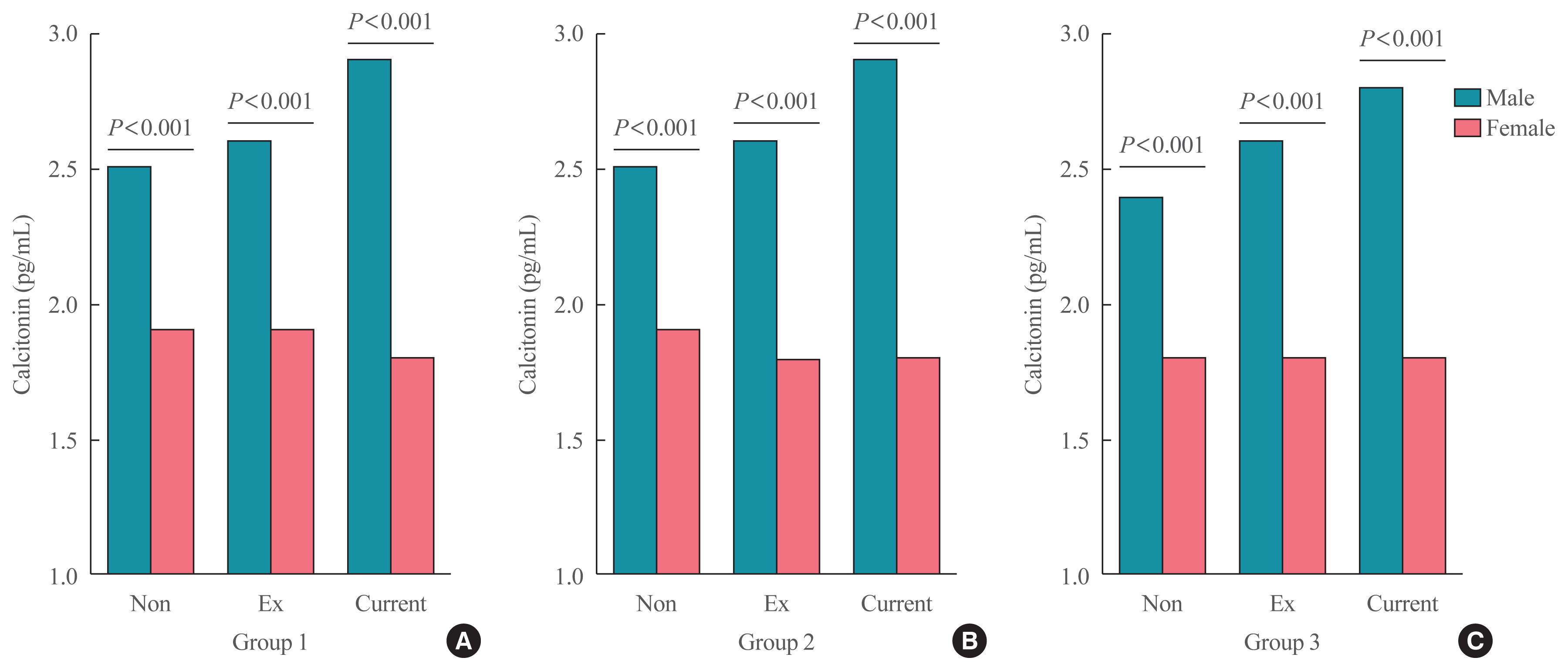
 XML Download
XML Download