Abstract
Sarcoidosis is a systemic granulomatous disorder of unknown etiology characterized by granuloma formation. Due to the limited incidence of sarcoidosis in pediatric patients, little is known about the clinical course of this disease. A combination of clinical, radiologic, and pathologic examination is necessary to exclude other differential diagnoses (i.e., infection and granulomatous inflammatory disorder) and establish a diagnosis of sarcoidosis. Here, we report a case of histologically confirmed sarcoidosis initially misdiagnosed as hepatosplenic abscesses in an 11-year-old male. Treatment with corticosteroids improved his symptoms and resolved his skin and hepatosplenic lesions. A three-year follow-up was uneventful. This study emphasizes the importance of considering sarcoidosis in children presenting with findings of multi-organ involvement in the presence of histologic evidence of granuloma.
Sarcoidosis is a systemic granulomatous disorder of unknown etiology. The clinical phenotypes range from self-limited and asymptomatic localized disease to high-risk multi-organ involvement. This disease mainly affects the lungs; however, any organ system of the body may be involved [1,2].
It is most common in young adults presenting with hilar lymphadenopathy, pulmonary infiltrates, and oculocutaneous lesions. In contrast, sarcoidosis in children is relatively rare. In children younger than 5 years, the disease involves the skin, eye, and joints, whereas in older children, the disease predominantly involves the lungs, lymph nodes, and eyes [1,3].
Due to the myriad phenotypes, there is no single test to diagnose sarcoidosis [2]. The presence of clinical, radiologic, and laboratory findings highly suggestive of sarcoidosis are not enough to establish the diagnosis as these tests are non-specific [4]. The diagnosis must be confirmed by histopathologic detection of typical granulomas on affected organs, provided that other granulomatous diseases, infectious etiologies, and immune deficiency have been ruled out [2,5]. Because sarcoidosis clinically and radiologically mimics other conditions, it is often misdiagnosed as an infectious, neoplastic, and inflammatory disease.
Here, we report a case of histologically confirmed sarcoidosis initially misdiagnosed as hepatosplenic abscesses in an 11-year-old male.
An 11-year-old male was admitted to our hospital for fever. His height was 153 cm (90~95 percentile), and weight was 56 kg (90~95 percentile). The vital signs at the time of admission were as follows: blood pressure, 115/74 mmHg; respiratory rate, 20 breaths/min; heart rate, 90 beats/min; temperature, 39.2°C. Two months prior to consult, the patient was hospitalized for a month due to fever and skin rash with multiple erythematous indurated nodules on lower legs (Figure 1A). Abdominal computed tomography (CT) performed 5 weeks before hospitalization showed multiple poorly defined small hypodense splenic lesions and a small hypodense hepatic lesion with mild peripheral enhancement, suggestive of abscesses (Figure 2A). Treatment with intravenous antibiotics and an antifungal agent improved his fever, skin rash, and hepatosplenic lesions on follow-up CT. He was discharged, and a course of oral antibiotics was prescribed. However, 10 days after discharge, his fever recurred, which prompted referral to our hospital.
Physical examination revealed enlarged cervical and axillary lymph nodes with hepatosplenomegaly. Other physical examination findings were normal. Initial blood tests revealed an elevated white blood cell (WBC) count of 33,820 cells/µL (normal range: 4,000~10,800 cells/µL), an elevated erythrocyte sedimentation rate (ESR) of 120 mm/h (normal range: 0~15 mm/h), and an elevated C-reactive protein (CRP) level of 132.8 mg/L (normal range: 0~8 mg/L) (Figure 3). Initial abdominal CT in our hospital on hospital day 1 (HD#1) showed resolution of the splenic abscesses and size reduction of the low-attenuating lesion at the right hemiliver (Figure 2B).
Empiric treatment with intravenous teicoplanin (340 mg/day, 6 mg/kg once daily) and meropenem (3,000 mg/day, 1 g every 8 hours) was administered from HD#1 to HD#24. After five days, his fever subsided. On the second week of treatment, his WBC count (14,000/µL), ESR (104 mm/h), and CRP (12.9 mg/L) were reduced (Figure 3). However, on HD#18, he experienced fever of 37.6°C and multiple painful, erythematous, indurated nodules in anterior aspect of the right lower leg (Figure 1B) with an associated elevation of CRP levels (45 mg/L). A follow-up abdominal CT on HD#18 revealed a slight size reduction of the low-density hepatic lesion (Figure 2C). Chest CT on HD#20 showed multiple small nodular lesions in both lungs (Figure 4A and 4B). Microbial cultures of the blood, urine, and stool revealed the absence of pathogenic bacteria and fungi. Interferon gamma release assay excluded tuberculosis. These findings suggested that his condition was likely non-infectious in origin.
Primary immunodeficiency work-up (i.e., immunoglobulin level, T and B cell count, T cell subsets by flow cytometry, and dihydrorhodamine test) was normal. Autoantibodies (i.e., anti-nuclear antibodies and anti-neutrophil cytoplasmic antibodies) were all negative (Table 1). A review of the skin biopsy slides prepared by the other hospital confirmed the presence of noncaseating epithelioid and giant cell granuloma (Figure 5). These findings were consistent with a diagnosis of pediatric sarcoidosis. Ophthalmologic evaluation revealed no ocular involvement, and level of angiotensin converting enzyme was 28 U/L (normal range: 29~112 U/L). Treatment with intravenous dexamethasone (15 mg/day) from HD#22 to HD#24 improved his fever and skin lesions with associated normalization of various inflammatory markers (Figure 3). Upon discharge, his medication was shifted to oral prednisolone (30 mg/day), which was tapered for over 11 months. Six months later in an outpatient clinic, no specific lesions were observed in chest (Figure 4C and 4D) and abdominal CT (Figure 2D). A three-year follow-up was uneventful.
This study was approved by the Institutional Review Board of Yonsei University Health System (IRB no. 4-2021-0548). The requirement of obtaining informed consent was waived by the board.
Sarcoidosis is a multisystem disorder that mainly affects adolescents and adults between the ages of 10 and 40 years with an overall prevalence of 10 to 20 per 100,000 people [4]. Sarcoidosis exhibited geographic and racial variation such that it was more common by about 3~4 times and more aggressive in blacks than in whites [6]. Moreover, the lifetime risk for sarcoidosis was higher in African Americans (2.4%) than in whites (0.8%) [4]. Although the true incidence and prevalence are unknown due to the limited reported cases in children, studies among Danish patients estimated an incidence of about 0.29 per 100,000 person-years in children younger than 15 years of age. The incidence of pediatric sarcoidosis ranged from 0.06 per 100,000 per year for children below 5 years old to 1.02 per 100,000 per year for children 14 to 15 years old [7]. Moreover, most of the pediatric cases were reported in children between 13 and 15 years of age. Although the prevalence rate was slightly higher in adult women, the gender predominance in pediatric cases remains unclear [1,3,8,9]. In terms of clinical presentation, extrapulmonary manifestations were more commonly observed in symptomatic pediatric patients than in adults [4].
Pediatric sarcoidosis appears to have two distinct clinical courses depending on the age of onset. Early-onset sarcoidosis in children under 5 years of age is characterized by a triad of rash, uveitis, and arthritis. In contrast, sarcoidosis in older children presents with lymphadenopathy, pulmonary infiltrates, and non-specific signs and symptoms (i.e., fever and malaise), similar to adult-onset sarcoidosis [1,8,10]. Our patient was an 11-year-old male presenting with fever, rash, lymphadenopathy, and radiologically detectable lesions on the lung, liver, and spleen, similar to the clinical patterns in adult-onset sarcoidosis. This suggests that our case was more consistent with the latter type.
The rarity of pediatric cases and considerable variations in clinical manifestations make the diagnosis of sarcoidosis difficult to establish [2,8]. Together with appropriate radiographic and clinical findings, histopathologic demonstration of typical non-caseating granulomas on biopsy specimen is necessary to confirm the diagnosis. Nevertheless, it is as important to exclude other potential etiologies such as infectious granulomatous conditions (e.g., fungal infection or tuberculosis), and granulomatous inflammatory disorders (e.g., Crohn’s disease, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and lymphomatoid granulomatosis) [1,4,8]. In our case, the diagnosis was established by skin biopsy. Biopsy of suspected lesions may be necessary in patients with systemic manifestations that cannot be explained by other causes.
To date, cases of pediatric sarcoidosis in Korea are limited. There are four reported cases of early-onset childhood sarcoidosis [11-14]. The first was a case of sarcoidosis in a 4-year-old female with the involvements of the skin and eye [11]. The second was a case of early onset childhood sarcoidosis in a 4-year-old female presenting with fever, right ankle joint swelling, multiple skin lesions on whole body and was misdiagnosed as juvenile rheumatoid arthritis [12]. The third was a case of pulmonary sarcoidosis in a 6-year-old female with no histopathologic confirmation due to the rapid disease progression [13]. The forth was a histologically confirmed case of sarcoidosis in a 33-month-old female with skin rash, subcutaneous nodules, and polyarthritis, clinically masquerading as Langerhans cell histiocytosis [14]. All of these were early-onset types of sarcoidosis. To the best of our knowledge, this is the first case of sarcoidosis in older children with multi-organ involvement diagnosed by biopsy.
Pediatric sarcoidosis can exhibit a variety of clinical manifestations. This study highlights that although the incidence of sarcoidosis is rare in children, it should be considered a differential diagnosis in patients with systemic symptoms in the presence of histologic evidence of granuloma.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: J.G.A. Data curation: S.M.L. Formal analysis: S.M.L., H.C., S.L., J.S., J.M.K., J.G.A. Investigation: S.M.L., H.C., J.G.A., Methodology: S.M.L., S.L., J.S., J.G.A. Project administration: J.G.A. Supervision: J.G.A. Writing—original draft: S.M.L. Writing—review & editing: J.G.A.
REFERENCES
1. Shetty AK, Gedalia A. 2000; Sarcoidosis in children. Curr Probl Pediatr. 30:149–76. DOI: 10.1067/mps.2000.105929. PMID: 10826082.

2. Chiu B, Chan J, Das S, Alshamma Z, Sergi C. 2019; Pediatric sarcoidosis: a review with emphasis on early onset and high-risk sarcoidosis and diagnostic challenges. Diagnostics (Basel). 9:160. DOI: 10.3390/diagnostics9040160. PMID: 31731423. PMCID: PMC6963233.

3. Fauroux B, Clément A. 2005; Paediatric sarcoidosis. Paediatr Respir Rev. 6:128–33. DOI: 10.1016/j.prrv.2005.03.007. PMID: 15911458.

4. Heinle R, Chang C. 2014; Diagnostic criteria for sarcoidosis. Autoimmun Rev. 13:383–7. DOI: 10.1016/j.autrev.2014.01.035. PMID: 24424172.

5. Nathan N, Sileo C, Calender A, Pacheco Y, Rosental PA, Cavalin C, et al. 2019; Paediatric sarcoidosis. Paediatr Respir Rev. 29:53–9. DOI: 10.1016/j.prrv.2018.05.003. PMID: 30917882.

6. Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. 1997; Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 145:234–41. DOI: 10.1093/oxfordjournals.aje.a009096. PMID: 9012596.

7. Rose CD, Wouters CH. Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, Mellins ED, Fuhlbrigge RC, editors. 2021. Pediatric sarcoidosis. Textbook of pediatric rheumatology. 8th ed. Elsevier;Philadelphia: p. 559–66. e2.
8. Gedalia A, Khan TA, Shetty AK, Dimitriades VR, Espinoza LR. 2016; Childhood sarcoidosis: Louisiana experience. Clin Rheumatol. 35:1879–84. DOI: 10.1007/s10067-015-2870-9. PMID: 25616361.

9. Hoffmann AL, Milman N, Byg KE. 2004; Childhood sarcoidosis in Denmark 1979-1994: incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr. 93:30–6. DOI: 10.1111/j.1651-2227.2004.tb00670.x. PMID: 14989436.

10. Shetty AK, Gedalia A. 2008; Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 6:16. DOI: 10.1186/1546-0096-6-16. PMID: 18811966. PMCID: PMC2559831.

11. Whang SW, Kim DS, Lee KH. 2000; A case of early-onset childhood sarcoidosis. Korean J Dermatol. 38:1696–8.
12. Jang KA, Choi JH, Sung KJ, Moon KC, Koh JK, Kim HK. 1998; Sarcoidosis in a four-year-old girl. Korean J Dermatol. 36:331–4.
13. Kim SA, Bae SY, Lee SY, Jeong DC, Chung SY, Kang JH. 2006; A case of pulmonary sarcoidosis in a 6-year-old girl. Pediatr Allergy Respir Dis. 16:253–8.
14. Lee JH, Lim YJ, Lee S, Joo KB, Choi YY, Park CK, et al. 2012; Early-onset childhood sarcoidosis with incidental multiple enchondromatosis. J Korean Med Sci. 27:96–100. DOI: 10.3346/jkms.2012.27.1.96. PMID: 22219622. PMCID: PMC3247783.

Fig. 1
Skin lesions. (A) The patient presented with skin rashes with multiple erythematous indurated nodules on both legs two months prior to consult. The figure is the lesions in the posterior part of the left lower leg. (B) The patient presented with multiple painful, erythematous indurated nodules in anterior aspect of the right lower leg on hospital day 18.
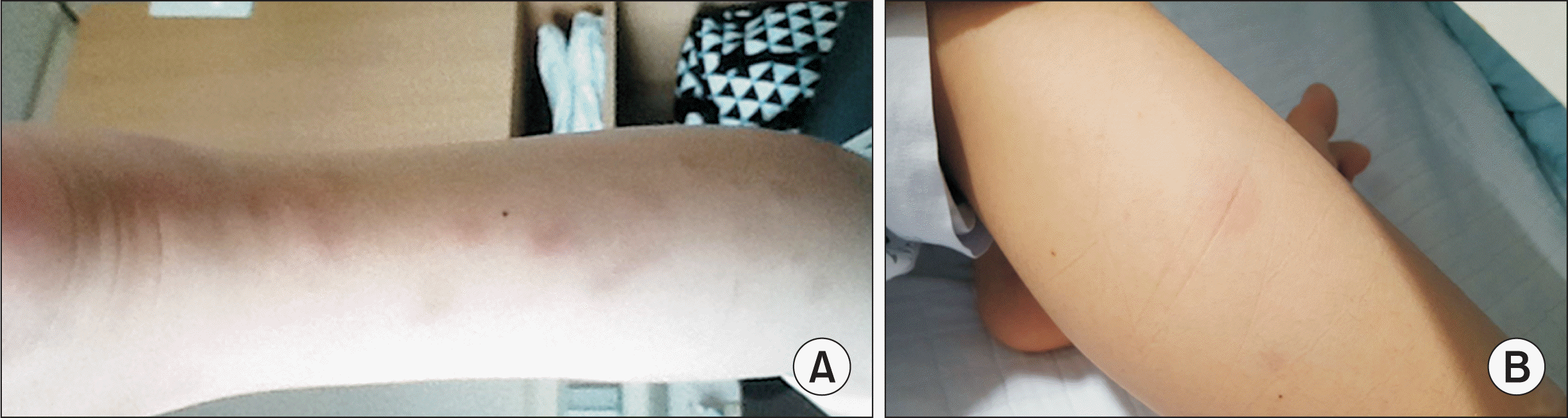
Fig. 2
Abdominal computed tomography (CT) findings. (A) CT taken at the other hospital 5 weeks before hospitalization showed multiple poorly defined small hypodense lesions in the spleen (white arrows), and a small hypodense lesion with mild peripheral enhancement in the liver (black arrow), suggestive of hepatosplenic abscesses. (B) Initial CT done in our hospital on the day of hospitalization revealed resolution of the splenic abscesses and size reduction of the low-attenuating lesion at the right hemiliver. (C) Follow-up CT on hospital day 17 showed further size reduction of the hepatic lesion. (D) Six months later in an outpatient clinic, no specific lesions were observed in abdominal CT.
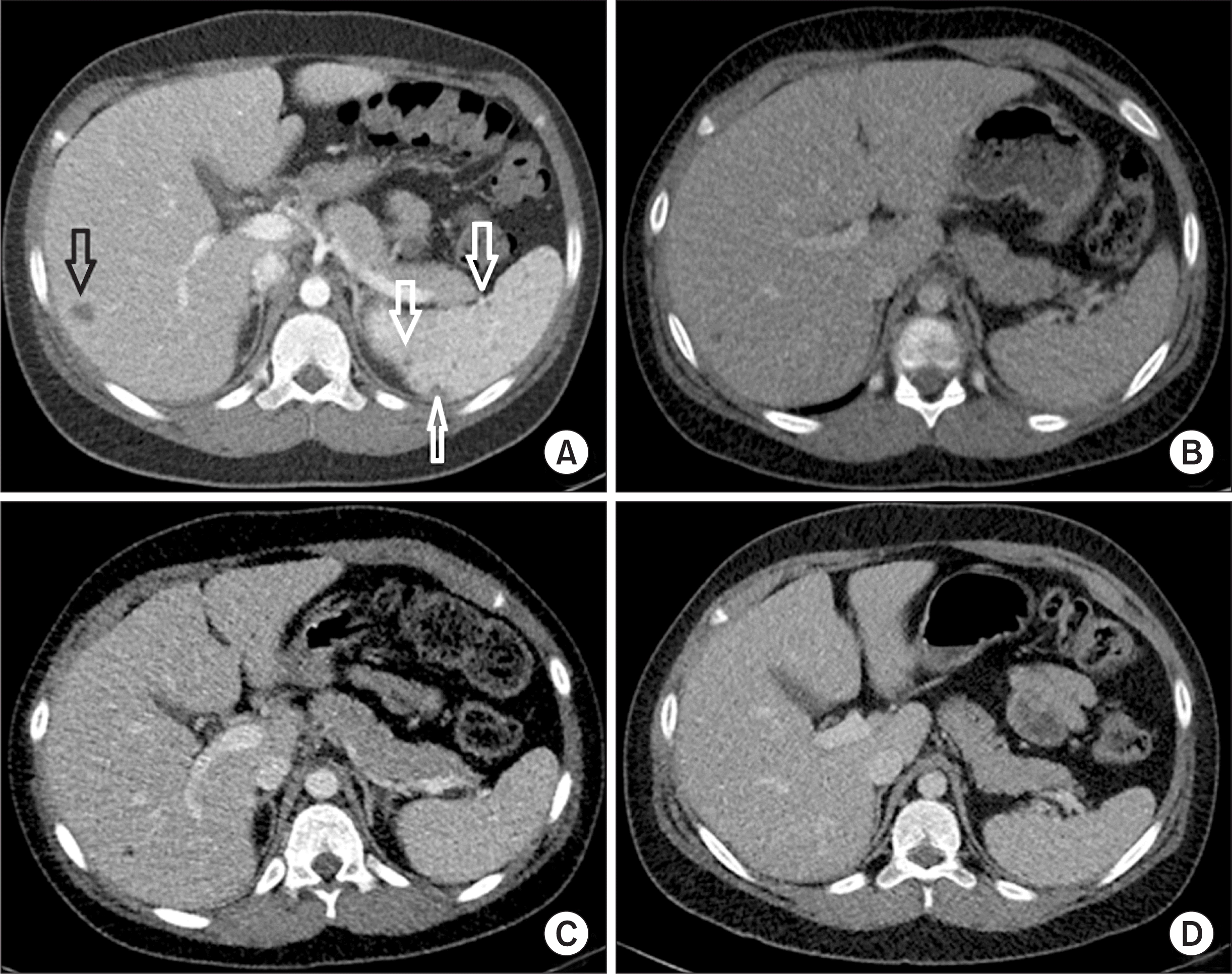
Fig. 3
Clinical time course of the patient. CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, WBC: white blood cell, IV: intravenous.
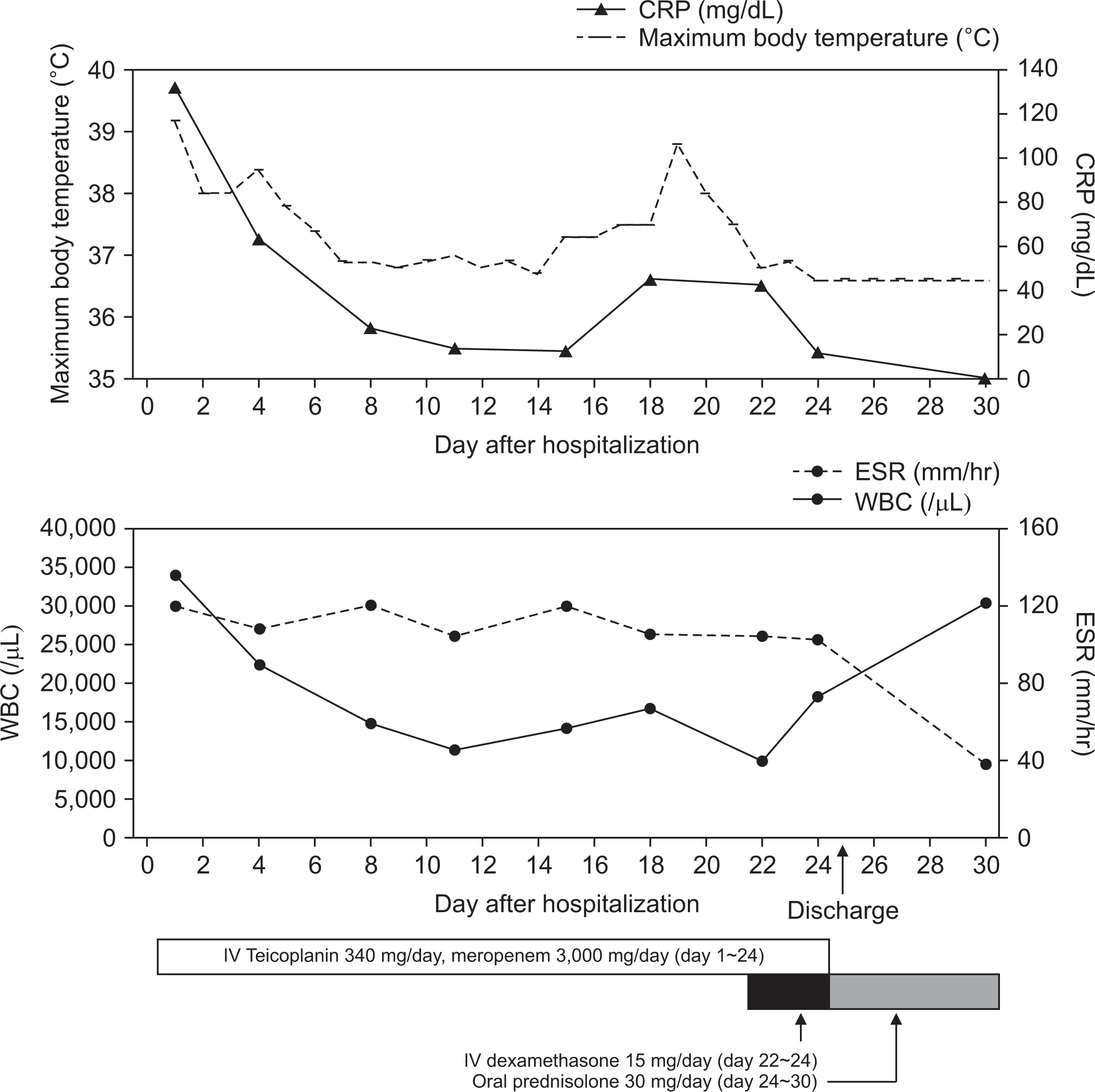
Fig. 4
Chest computed tomography (CT) findings. (A, B) Chest CT on hospital day 20 revealed multiple small nodular lesions in both lungs (arrow). (C, D) Six months later in an outpatient clinic, no specific lesions were observed in chest CT.
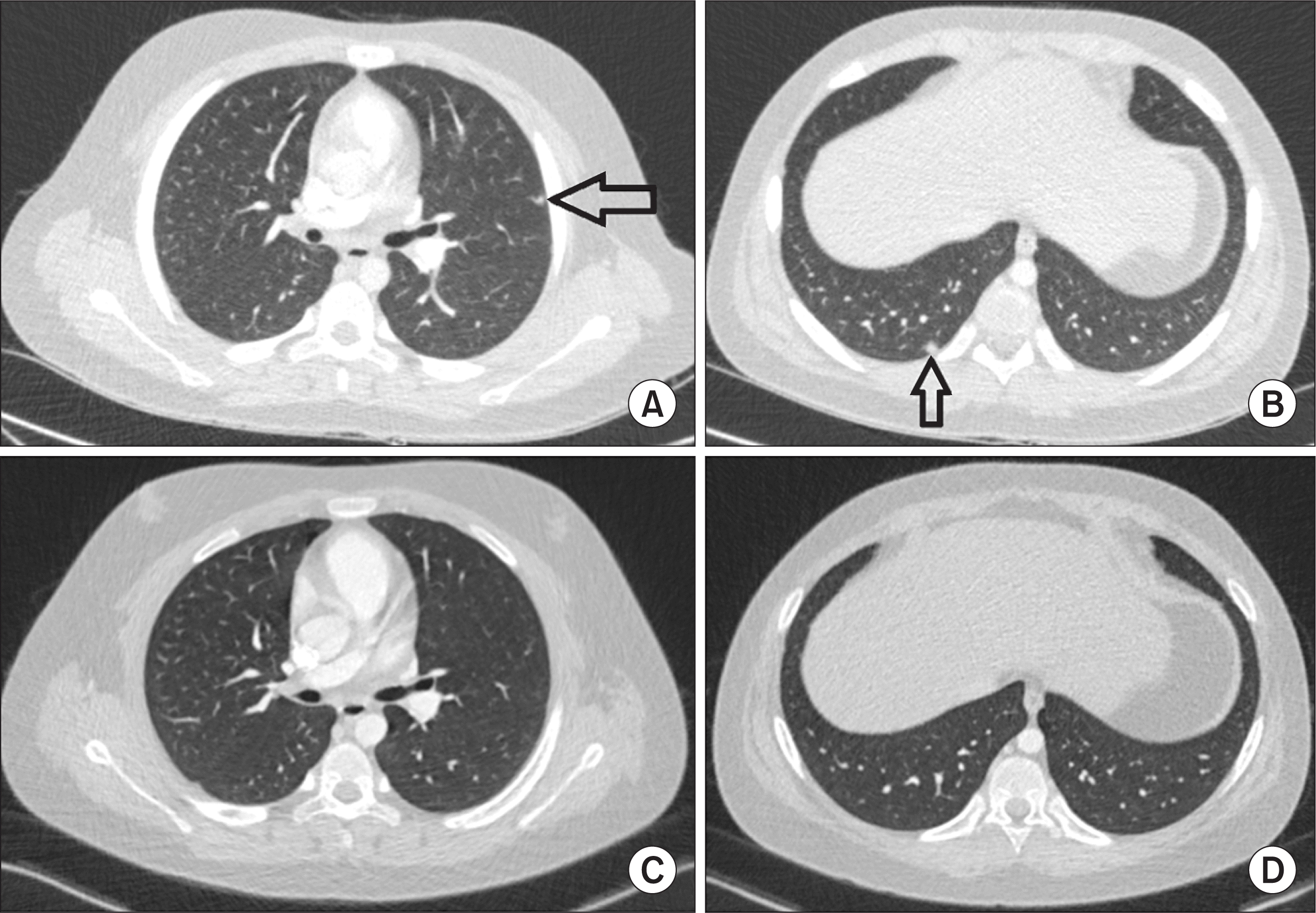
Fig. 5
Histopathologic examination of skin biopsy specimen from the right lower leg. (A) Infiltration of mixed inflammatory cells (lymphocytes, histiocytes, plasma cells, etc.) is observed in subcutaneous fat lobules (arrows in A) (H&E stain, x100). (B) Noncaseating epithelioid and giant cell granuloma are noted (arrows in B) (H&E stain, x200).
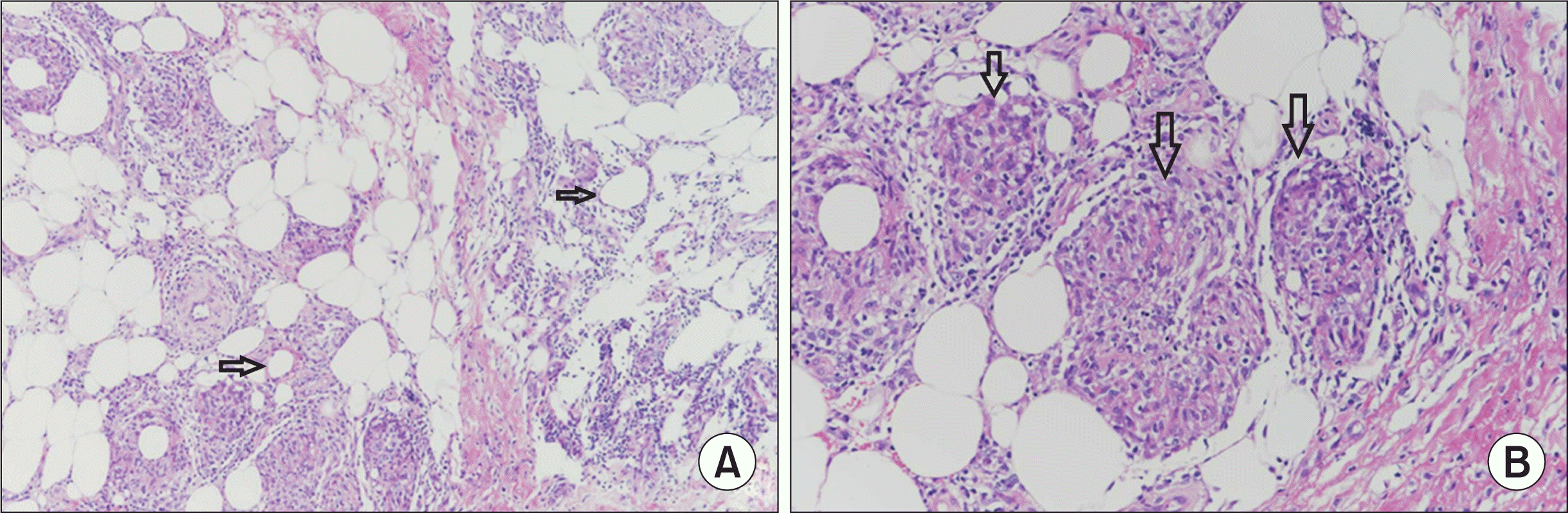
Table 1
Immunological test results of the patient




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download