Abstract
Background/Aims
Methods
Results
Conclusions
Notes
Author Contributions
Conceptualization: Jun Heo, Minkyu Jung, Chang Min Cho
Data curation: Sun Young Moon
Formal analysis: SYM
Investigation: SYM, JH, MKJ, CMC
Methodology: SYM, JH, MKJ, CMC
Project administration: JH
Resources: JH, MKJ, CMC
Supervision: JH, MKJ
Validation: JH, MKJ, CMC
Visualization: SYM
Writing-original draft: SYM
Writing-review&editing: SYM, JH, MKJ, CMC
REFERENCES
Fig. 1.
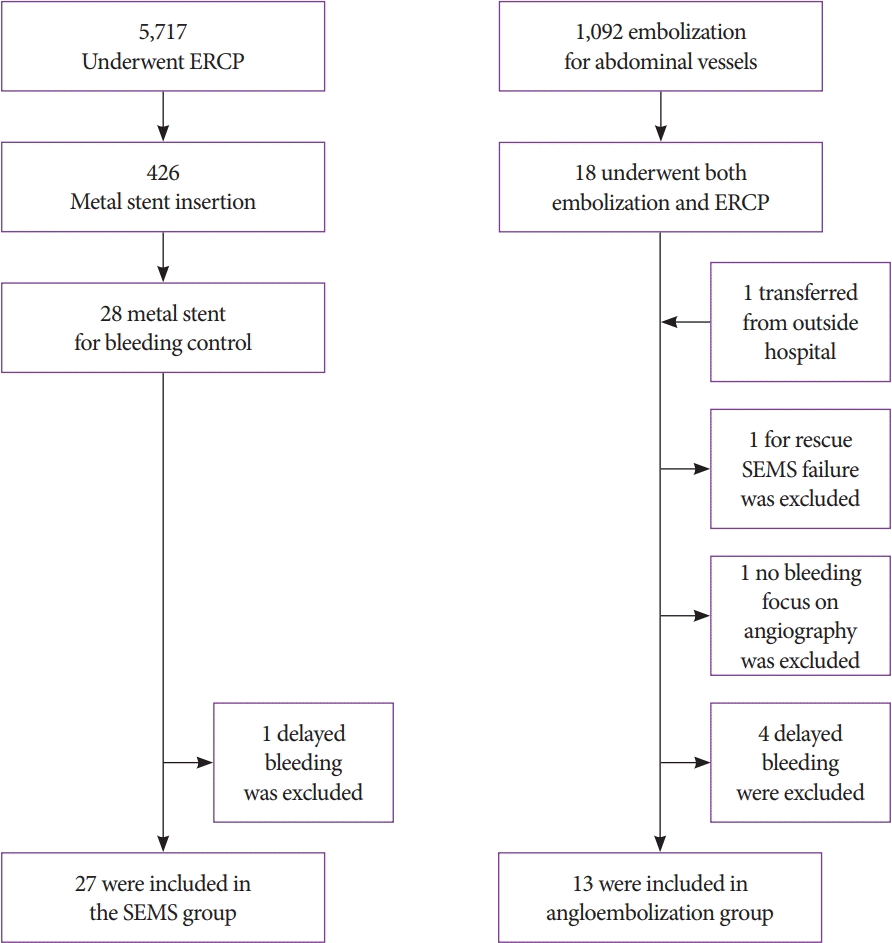
Table 1.
Data are presented as the number (%) or mean±standard deviation.
ALT, alanine transaminase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CCI, Charlson comorbidity index; ERCP, endoscopic retrograde cholangiopancreatography; Hb, hemoglobin; PB, pancreatobiliary; PT, prothrombin time; SEMS, self-expandable metal stent; WBC, white blood cell.
Table 2.
| SEMS (n=27) | Angioembolization (n=13) | p-value | |
|---|---|---|---|
| ERCP procedure time (min) | 23.8±7.4 | 21.4±7.3 | 0.336 |
| Post-ERCP pancreatitis cases | 0 (0) | 1 (7.7) | 0.144 |
| Bleeding severity | 0.006 | ||
| - Mild (no transfusion) | 15 (55.6) | 3 (23.1) | |
| - Moderate (upto 4 units) | 12 (44.4) | 6 (46.2) | |
| - Severe (>5 units) | 0 (0.0) | 4 (30.8) | |
| Shock, before ERCP | 3 (11.1) | 2 (15.4) | 0.702 |
| Using inotropics | 3 (11.1) | 3 (23.1) | 0.321 |
| Other ERCP-related complications | 2 (7.4) | 1 (7.7) | 0.974 |
| Bleeding during ERCP | 0.792 | ||
| - Post-ES bleeding | 15 (55.6) | 6 (46.2) | |
| - Post-EPLBD bleeding | 9 (33.3) | 6 (46.2) | |
| - Other | 3a) (11.1) | 1b) (7.7) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download