Abstract
The survival rate of cancer is increasing as treatment improves. As patients with cancer now live longer, impairments may arise that impact quality of life (QOL) and function. Therefore, a focus on QOL is often as important as survival. An interdisciplinary team can achieve goal-oriented and patient-centered rehabilitation, which can optimize function and QOL, and minimize impairments, restrictions, and activity limitations. In most cases, cancer patients must be active participants in therapy and exhibit carryover. Patients with cancer often have impairments that include fatigue, pain, brain fog, impaired cognition, paresis, mood disorders, difficulty with activities of daily living (ADL), bowel/bladder/sexual dysfunction, and bone and soft tissue involvement. Adaptive equipment, exercise, and ADL training can mitigate restrictions on activity. The trajectory and phase of the disease along the continuum of cancer care may influence the goals of rehabilitation in that time window. QOL is often influenced by participation in vocational, recreational, and home-based activities. A holistic perspective should include an analysis of distress, socioeconomic barriers, and transportation limitations when addressing issues.
Rehabilitation is defined by the National Cancer Institute Dictionary of Cancer Terms as “a process to restore mental and/or physical abilities lost to injury or disease, in order to function in a normal or near-normal way” [1]. Cancer survivors and those afflicted with terminal disease rely on rehabilitation to optimize quality of life and maintain dignity. Rehabilitation takes a holistic approach integrating multiple key concepts. First, an approach that is “patient-centered”— tailored to the patient’s individualized situation, needs, and wants. The patient (and their family) is the leader of the team and carefully honors the patient’s wishes. Communication is key to ensuring that the healthcare team is up-to-date with a patient’s goals and the potential evolution of those goals across the cancer continuum. Second, a “goal-oriented” technique should be used for rehabilitation. Goals should be measurable, achievable, concrete, and typically focus on function and/or quality of life (QOL), thus allowing communication between the patient, caregiver, and the healthcare team. Hope should be maintained when discussing goals; however, it is also important to be realistic and discuss alternatives that maintain dignity. Third, rehabilitation that employs an “interdisciplinary team approach.” Common goals are paramount. Unlike tradiAnnals tional multidisciplinary teams, which strive to achieve specialty-oriented tasks, interdisciplinary team members work together to meet the patient’s goals as well as the objectives for their individual disciplines. Group communication, creativity, and humility are compulsory characteristics of team members needed to achieve these goals. Fourth, in most cases, “active participation of the patient” is necessary for rehabilitation. At times, the family may learn how to safely care for their loved one at the end of their life. However, during the majority of the cancer continuum, rehabilitation requires active engagement of the patient and family to partake in education, therapy, and display carry-over of newly acquired skills. There is a partnership between the healthcare team and patient; the patient takes on the onus of self-management.
The International Classification of Functioning, Disability, and Health (ICF), which supplements the International Classification of Disease (ICD-10), was created by the World Health Organization [2]. Definitions are listed in Fig. 1, which illustrates the integral role of rehabilitation in optimizing QOL. This review will analyze impairments, activity restrictions, and participation limitations in cancer patients and reveal the benefits of rehabilitation in mitigating disabilities.
The number of cancer survivors continues to grow because more people live longer with cancer as a result of new advances in surgery, medicine, and radiation oncology. Survival rates for all cancers in high income countries range from 52.7% in England, 66.6% in the United States, and 70.6% in Korea [3,4]. Thus, patients live longer with more years of cancer-related disability. In 2020, cancer caused 208.3 disability-adjusted life years (DALYs), as estimated by the Global Burden of Disease Cancer [5]. Impaired activities of daily living (ADLs) and mobility, as well as higher self-reported pain scores, were noted in cancer survivors over 55 years old compared with controls [6]. Improving survival rate is insufficient without also optimizing QOL for cancer survivors. Patients with lymphedema may have an impaired QOL. Approximately 200 million people are affected by lymphedema worldwide [7].
Patients with cancer benefit from rehabilitation at all phases of the cancer continuum (Table 1). As early as possible, clinicians should ask, “Has your function changed?” Given the complexity of cancer and chronic illness, a preventive and comprehensive strategy for disability and illness should be employed. Throughout the continuum of cancer, survivors are concerned about fatigue, overall health, pain, fitness, and social and emotional functioning [8]. Early in the disease, anxiety may be the most concerning symptom that is typically accompanied by the disruption of routines. Patients with cancer in the treatment phase, additional impairments, including nausea, fatigue, and sleep disturbances, may arise. Referral to cancer rehabilitation should occur if there is pain, fatigue, impaired ADLs and/or mobility. Additional guidelines that indicate site-specific referral recommendations have been published [9].
Physical function is likely to decline from baseline. At the time of initial cancer diagnosis, patients are likely to have physical impairments and ideally would benefit from “prehabilitation” [10]. Research has shown that education and exercise before treatment can reduce morbidity and decrease length of hospital stay [11]. In patients with vestibular schwannomas, preoperative vestibular training improves postoperative postural function [12]. Patients with the poorest endurance/lowest anaerobic threshold benefit the most from preoperative rehabilitation [13].
Physiatrists, who specialize in physical medicine and rehabilitation, can assist medically complex patients in diagnosing and treating impairments, and can prescribe racing, assistive devices, durable medical equipment, orthotics, injections, and medications. If required, precautionary measures can be taken. When needed, physiatrists can discuss the need for rehabilitation at home, or as part of outpatient or inpatient care [14].
Cancer rehabilitation focuses on diagnosis and treatment of impairments, which may affect multiple aspects of an individual’s function and QOL.
The vast majority of patients with cancer develop pain that may be debilitating during the course of their illness. This pain can arise from the tumor itself, the sequelae of chemotherapy, radiation, surgery, and/or related side effects [15]. Function is intimately correlated with the severity of pain, and this was demonstrated in 216 patients with metastatic cancer [16].
Cancer-related fatigue has been defined as “overwhelming and sustained exhaustion and decreased capacity for physical and mental work…not relieved by rest” [17]. Exercise can reduce cancer-related fatigue [18]. Emotions and social and economic status can have negative impact on fatigue [19]. Cancer-related fatigue can be reduced by optimizing sleep quality; however, increasing “rest” is not helpful [20].
Up to 86% of advanced cancer patients have high incidence of delirium [21]. About 50% of the time, delirium may be reversible and due to manageable and identifiable causes [22]. Appropriate diagnosis is needed to determine the appropriate treatment, and there are numerous causes of delirium in patients with cancer. Research on bone marrow transplant patients has demonstrated risk factors in pre-transplant patients with higher blood urea nitrogen, magnesium, and alkaline phosphatase levels, and lower cognitive/physical function [23].
Medication plays a significant role in the development of delirium. In a study of 216 hospitalized cancer patients, corticosteroid, opioid, and benzodiazepine use was more frequently associated with delirium than the use of other drugs [23]. The clinician must also consider metabolic factors. Fever and sepsis often cause acute delirium, and dehydration and uremia frequently contribute to this condition. Hypoxia and hypoglycemia are additional factors that can be easily assessed.
Delirium may present as either a hypoactive or a hyperactive state. Dehydration is a frequent contributing factor to hypoactive cases. Adverse effects of medications (especially opioids and corticosteroids) and liver failure are often implicated in hyperactive states.
Patients with cancer often report cognitive difficulties following chemotherapy and other treatment regimens. However, one study indicated no significant differences in the long-term cognitive function of cancer survivors and control subjects [24]. Many of these reports on cognitive difficulty may be related to fatigue.
Receiving a cancer diagnosis is stressful and frightening for most individuals. Initially, they may experience symptoms of shock, disbelief, denial, or despair, as they struggle to accept and incorporate the reality of the diagnosis. Patients may also experience various normal fears throughout their treatment course, including fear of disability, loss of societal roles, loss of control, loss of desirability, abandonment, and death. However, most patients cope successfully with cancer diagnosis and treatment and experience good long-term psychological adjustment. Many patients even described positive changes in their lives related to their diagnosis, including positive changes in self-perception, interpersonal relationships, priorities, and goals.
Although most patients cope well, a significant number experience serious mood disorders. Estimates of the prevalence of depression among patients with cancer range from 15% to 25% [25]. Anxiety is common and may be related to poorly controlled pain, abnormal metabolic states, or medication-related adverse effects. Patients may also experience post-traumatic stress disorder (PTSD) in response to cancer diagnosis and treatment. PTSD is an anxiety disorder that develops after an extremely stressful event, such as life-threatening illness. Within 5 years of diagnosis, between 10% and 15% of cancer survivors may meet the criteria for PTSD [26].
A wide variety of impairments of nervous system function may result from cancer, either by direct effects at the primary or metastatic tumor site or secondarily as a consequence of surgical or radiation treatment. These impairments, regardless of the tumor’s location, extent, or type, may have an adverse impact on an individual’s physical, social, vocational, and emotional capabilities. Important differences exist between the management of patients with cancer of the central nervous system and those with other types of acquired neurological disabilities.
Brain tumors vary widely in terms of their aggressiveness and prognosis. The extent to which tumor type or location has an impact on rehabilitation outcomes is unclear. However, one study found a tendency for better rehabilitation outcome in patients with meningiomas and left-hemispheric lesions. Patients receiving acute inpatient rehabilitation show similar improvement regardless of whether they have a primary brain tumor or a brain tumor resulting from metastatic disease. Some studies have shown that patients with brain tumors have shorter lengths of stay in acute rehabilitation units than do patients with other noncancerous brain disorders [27].
Most patients with brain tumors have multiple impairments depending on the tumor location, size, and volume of tissue excised during surgery. In a study of patients undergoing acute rehabilitation, the most common neurological deficits included impaired cognition (80%), weakness (78%), and visual-perceptual dysfunction (53%) [28]. Rehabilitation efforts should focus on the patient’s neurological and functional status, coexisting medical problems, and tolerance of physical activity. For patients who have had a stroke or traumatic brain injury, goal setting should be appropriate to the individual’s physical, cognitive, and behavioral status and should include early planning for post-acute rehabilitation care.
The incidence of cancer-related spinal cord injury (SCI) may exceed that of trauma, and represents the most frequent type of non-traumatic SCI [29]. Spinal cord metastases produce a clinical syndrome characterized initially by pain in 90% of cases, followed by weakness, sensory loss, and sphincter dysfunction. Weakness is present in 74% to 76% of patients, autonomic dysfunction is present in 52% to 57% of patients, and sensory loss is present in 51% to 53% of patients [30].
Disorders of speech and swallowing may be the result of direct tumor invasion of the oral cavity, larynx, pharynx, esophagus, or adjacent structures, or the result of surgical or radiation treatment, or they may be a consequence of nervous system cancers that affect pharyngeal or laryngeal control. Head and neck cancers constitute approximately 3% to 5% of all malignancies. Preservation of swallowing, having a natural airway, and intact speech are critical components that affect the QOL of patients with head and neck cancer. Among these patients, swallowing has been shown to have the largest impact on global QOL [31].
As soon as feasible after surgery, oral motor exercises should be initiated by speech/language pathologists with a focus on the strength, range of motion, and sensory awareness of the involved structures. Common interventions include modifying food texture, such as thickening liquids or purifying solid food, and/or altering head posture and swallowing behavior. The latter may include techniques such as a chin tuck to prevent laryngeal penetration, head rotation to reduce retention in the piriform sinus, enforced double or effortful swallowing to reduce pharyngeal residue, or supraglottic swallowing to optimize vocal fold closure and airway clearance.
In most patients with head and neck cancer, impaired vocal communication occurs during treatment. It is important to keep in mind that multiple conditions other than total laryngectomy can result in deficient phonation in cancer patients. These conditions include copious secretions, localized edema, fibrosis and scarring, tracheostomy, glossectomy, loss of oral mobility from local tumor or trismus, and neurogenic pharyngeal or laryngeal paralysis. Patients who have undergone total laryngectomy lack a source of voice production and need to replace laryngeal function with an artificial larynx (electrolarynx), esophageal speech, or tracheoesophageal puncture voice restoration with a prosthetic surgical device.
Soft tissue and bony sarcomas are managed using amputation or limb-sparing procedures. Limb salvage procedures are increasing in frequency and are associated with long-term survival, local recurrence rates, and QOL equivalent to that of patients with amputations. These procedures are largely made possible by improved surgical techniques that preserve unaffected tissue, advances in endoprosthetic design and durability, soft tissue reconstructive procedures, and effectiveness of radiation and chemotherapy in controlling local and distal spread.
Rehabilitation after limb-sparing procedures depends on the extent of soft tissue and bony resection, and because of the nature of tumor resection, skeletal reconstruction, and soft tissue and muscle transfers, rehabilitation may be more intensive than after amputation. Rehabilitation includes instruction regarding the use of mobility aids and orthotics for joint stabilization and assistance with strengthening and endurance exercises in collaboration with the treating surgeon.
Bone metastases are a frequent source of cancer-related physical impairment that require active involvement of the rehabilitation team. Challenges for the treatment team arise when metastatic bone lesions produce severe pain that limits function or imposes risks of fracture during therapeutic exercise or mobility. The incidence of pathological fractures among all tumor types is approximately 8%, and breast carcinoma is responsible for the majority of these fractures. Sixty percent of all long-bone fractures involve the femur, with most of these fractures involving the proximal portion [32]. If a patient is deemed at risk of a pathological fracture, they should not bear weight on the affected structure, pending an orthopedic consultation.
Rehabilitation of this patient population focuses on removing weight from or immobilizing compromised bone through the provision of assistive devices and orthoses, strength and balance training, and modification of the patient’s environment. Whenever possible, bed rest should be avoided because it adds to general debility and further functional loss, and also increases the risk of hypercalcemia and thromboembolic disease. It is critical to rule out the co-existence of upper extremity lytic lesions before prescribing assistive devices that require weight support through the arms. Bracing may reduce the risk or symptoms of pathological fractures involving the upper extremities and can facilitate the use of the arms in functional activities. Individuals with upper limb lesions should be taught to minimize torsion and weight loading and may benefit from an arm sling or humeral cuff support. In the spine, when more rigid bracing is not possible because of poor skin tolerance or discomfort, the thoracolumbar corset provides limited support and pain relief. Patients with cancer who experience pathological fractures and associated functional deficits have shown significant gains when admitted to an inpatient rehabilitation hospital unit [33].
Cancer, its treatment, or both can cause significant soft-tissue abnormalities. One of the most frequently observed abnormalities is lymphedema, that is, extremity swelling that results from disruption of the lymphatics after axillary or groin dissection. Lymphedema can become debilitating. In a systematic review, lower extremity lymphedema was associated with significantly reduced QOL [34]. The use of manual lymph drainage and compression garments is effective in controlling edema. When applied early in the course of treatment, before the development of a significant volume increase (e.g., >250 mL increase in the arm), lymphedema can be reversed [35]. Traditionally, patients with lymphedema have been told not to lift weights. However, new data show that not only does weightlifting not worsen lymphedema, but it may also be beneficial [36].
Radiation fibrosis is another frequently observed complication of cancer treatment [37]. This process is associated with vascular permeability, inflammation, and the release of proinflammatory cytokines (e.g., interleukins and transforming growth factor-β), and continues well past cessation of radiation therapy. Physical therapy is particularly important for maintaining range of motion. Splinting may also be helpful in certain cases. The use of antifibrotic agents for the treatment of this condition has shown promise [38].
Allogeneic bone marrow transplantation has prolonged life expectancy for many patients with hematologic malignancies. One of the complications of this procedure is rejection of the host by the transplanted, immunocompetent engrafted cells, called graft-versus-host disease. The immunological reaction is often brisk, resulting in organ damage (fibrosis) to the lung, liver, skin, and soft tissue. In the chronic form of graft-versus-host disease, limb edema, peau d’orange, fasciitis, and enthesitis can occur, resulting in significant loss of joint motion. Subsequent muscle atrophy may occur as a result of disuse and the associated loss of upper and lower extremity mobility [39].
Loss of bladder or bowel control in patients with cancer is often multifactorial and can be a result of neurogenic causes, such as with brain or spinal cord tumors; it also can result from nonsurgical or surgical cancer therapies or can occur as a direct effect of gastrointestinal or genitourinary tumors. Incontinence may also be related to immobilization in the bed, adverse effects of pharmacological management, or diminished alertness and communication skills. This condition encompasses both medical consequences and QOL issues; it is incumbent on the treatment team to properly assess patients with fecal or urine incontinence and institute a management plan.
Maintaining sexual function can be very important for patients with cancer; however, it is seldom discussed by patients and physicians. In patients with breast cancer, treatment often produces adverse effects, such as fatigue, nausea, and diminished vaginal lubrication, in addition to the significant body image changes that accompany mastectomy. A meta-analysis of 36 studies of sexuality in patients with testicular cancer showed that problems were largely related to ejaculatory dysfunction, but fortunately, the rates of decreased sexual desire were low and often improve with time [40]. Erectile dysfunction is a common adverse effect of prostatectomy, hormonal therapy, and radiation therapy in prostate cancer patients. Surgery for colorectal cancer often leads to sexual dysfunction in men, and gynecological cancers often produce changes in vaginal sensation, structure, and lubrication. However, 63.5% of patients with cancer who received brief sexual counseling reported improvement [41].
It is not surprising that many people with cancer have significant limitations in their activities of daily living. These limitations include reduced mobility and limited ability to perform ADLs. ADLs often result from neurologic or orthopedic impairments but may also be related to fatigue. Communication and socialization skills may be adversely affected by cognitive deficits, speech impairments, depression, and anxiety.
Basic ADLs include feeding, dressing, hygiene, and toileting. These ADLs are universal to human dignity throughout the world. Impairments in upper limb function play an obvious role in limiting the performance of ADLs, but other impairments can also impede these functions. Cognition is critical for sequencing, awareness, and carryover in ADL performance. Pain and fatigue can limit an individual’s ability to complete these tasks. Lower limb impairments can limit standing and transfer, which makes dressing, hygiene, and toileting difficult.
When people become disabled enough to require assistance with these skills, the burden generally falls on caregivers. In one study of 483 patients with cancer at varying stages of their disease course, 18.9% had unmet needs in their ADLs because of the lack of a suitable caregiver [42]. In patients with advanced-stage cancer, the percentage of caregivers with a high level of psychological distress varied from 41% to 62%, directly depending on the functional status of the patient [43].
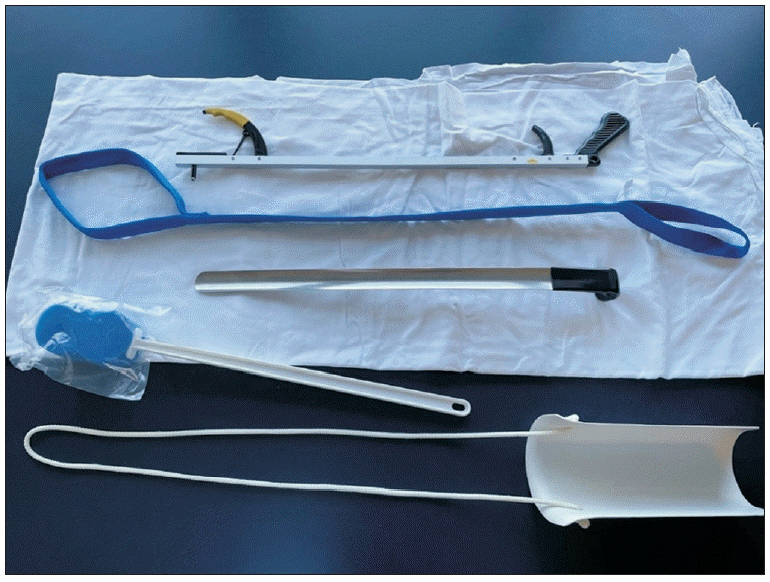
Rehabilitation efforts, particularly with the involvement of occupational therapy, can significantly reduce this burden on caregivers and enhance the QOL of cancer patients with disabling impairments. Addressing functional loss from impairments of the upper limb, such as chemotherapy-related peripheral neuropathy of the hand or radiation-induced brachial plexopathy, can substantially improve ADL performance. Simple adaptive aids (Fig. 2) can help patients perform everyday tasks. To improve feeding independence among patients with cancer who have upper limb neurologic dysfunction, Chinese researchers used positioning, feeding aid support, and upper limb support, and significantly improved function during a 3-week treatment intervention [44]. Homebased occupational therapy interventions produce a high level of patient and caregiver satisfaction, thereby reducing the burden of care.
Exercise is one of the most effective strategies for treating symptoms associated with cancer fatigue, sleep disruption, and abnormalities in mood, physical function, and QOL [45]. Meta-analyses [46] suggests that for adults with a variety of cancer diagnoses and who are receiving a variety of exercise interventions, exercise improves physical function, QOL, and cardiorespiratory fitness, and decreases cancer-related fatigue [47-49]. The majority of these studies employed aerobic exercise, ergometry and walking programs, and occasionally, aquatic therapies. Table 2 outlines the contraindications for exercise.
Physical modalities may be used to control pain and improve range of motion, thus leading to better mobility. Most physical modalities have not been well studied in patients with cancer because of concerns regarding the exacerbation of an underlying malignancy. Physical modalities that are generally believed to be safe include cryotherapy, biofeedback, iontophoresis (i.e., transdermal delivery of medication by electrical current), transcutaneous electrical nerve stimulation, and massage, which should not be used directly over a tumor site. Deep heat (e.g., ultrasound and phonophoresis) is usually contraindicated in patients with cancer because of the theoretical risk of metastasis from hyperemia. Spinal traction is contraindicated in patients with spinal metastases or significant osteoporosis.
Durable medical equipment is an important tool for improving the activity levels of patients with cancer. Durable medical equipment includes hospital beds, canes, walkers, wheelchairs, and motorized scooters. Wheelchairs should be individually fitted because using the wrong size can lead to skin breakdown or make accessibility difficult if the wheelchair is too wide. Oxygen supplementation can enhance endurance and cognition and reduce dyspnea in patients with hypoxia as a result of lung cancers or metastases.
Cancer can often draw a family together; however, this can also lead to significant distress. Support groups can be helpful; however, fewer than half of the patients receive information about them, even in large tertiary oncology centers [50]. In one study of 121 patients with cancer, caregiver QOL was significantly correlated with the social/family and functional dimensions of patients’ QOL; physical and emotional dimensions did not correlate [51]. Cancer also can place a significant economic burden on families. Cost considerations play a large role in patient decision-making regarding cancer treatment, especially among the poor.
Work disability after a cancer diagnosis is common. Short and Vargo [52] conducted phone interviews with 1,433 cancer survivors 1 to 5 years after diagnosis. More than half quit work during the first year after cancer diagnosis, but three-quarters of those subsequently returned to work. A projected 13% had indefinite work disability. Survivors of central nervous system, head and neck, and stage IV blood and lymph malignancies had the highest risk of quitting work.
In recent years, the United States laws have provided more protection for cancer survivors returning to work. These laws include the Americans with Disabilities Act, the Family and Medical Leave Act, and the Health Information and Portability Act. Nevertheless, a number of barriers remain in the way of gainful employment for cancer survivors with disabilities. These barriers include ignorance on the part of both employers and cancer survivors regarding their rights, discrimination, and limits on pre-existing health insurance benefits.
Little is known about the medical impairments that have the greatest impact on employability. Undoubtedly, cognitive and communication deficits play a large role, as evidenced by the high work disability rates among survivors of central nervous system and head and neck malignancies. Fatigue and pain are likely to limit work participation. Spelten et al. [53] studied 235 cancer survivors in the Netherlands and found that fatigue levels strongly predicted inability to return to work.
Recreation is critical for both physical and mental wellbeing. Fatigue, pain, weakness, depression, and other impairments can limit cancer survivors’ participation in vocational pursuits. The benefits of recreational activities include improvements in fitness, musculoskeletal problems, immune system function, cognition, and sleep quality. One study examined 97 European youth attending a summer camp for adolescents with cancer and diabetes [54]. Significant improvements in self-esteem, selfefficacy, and anxiety were observed. Adults benefit as well. For example, 11 small studies have shown that Tai Chi Chuan, an Asian mind-body practice, has beneficial effects in cancer survivors [55].
Patients with cancer may have a limited ability to drive, fly, or use public transportation. They may not have caregivers available to help transport them. A lack of transportation can become a major barrier to cancer treatment, which often involves frequent medical visits. Some patients forgo recommended treatments because of a lack of adequate transportation; thus, physicians should explore with patients any limits to their ability to use transportation. Galski et al. [56] showed that patients receiving chronic, stable opioid analgesic therapy could drive safely. Patients with cerebral dysfunction resulting from a tumor, paraneoplastic effects, or treatment adverse effects should be evaluated for their ability to drive safely before they are allowed to return to the road. Welldefined off-road driver evaluation tools are available.
Cancer rehabilitation is safe and prevents many medical complications. Nonetheless, cancer rehabilitation providers should be aware of several urgent medical conditions that require immediate intervention [57].
• Spinal cord compression presents as back pain and weakness. Imaging should be performed as soon as possible with referral to neurosurgery and/or radiation oncology.
• Cerebral edema presents as obtundation, cranial nerve palsies, and/or increased weakness. It also requires urgent imaging, with referrals to neurosurgery or radiation oncology.
• Hypercalcemia is characterized by drowsiness and change in mental status with hyporeflexia. Therefore, medical oncology should be contacted urgently.
• Venous thromboembolism presents as calf swelling and tenderness (deep venous thrombosis), tachycardia, and tachypnea (pulmonary embolism). Anticoagulation should be urgently initiated.
• Malignant pericardial effusion presents with chest pain and dyspnea. Echocardiography can confirm the diagnosis with appropriate referral to cardiology.
• Superior vena cava syndrome presents with sudden swelling of the neck, face, and limbs with dyspnea. It usually shows on chest radiography, but sometimes a chest CT is required.
• Neutropenic fever is life threatening and needs immediate attention for control of possible sepsis.
• Tumor lysis syndrome can result in life-threatening electrolyte abnormalities, particularly hyperkalemia, following chemotherapy.
REFERENCES
1. National Cancer Institute. NCI dictionary of cancer terms [Internet]. Washington, DC: National Cancer Institute. c2022 [cited 2022 Apr 15]. Available from: http://www.cancer.gov/dictionary.
2. World Health Organization. International classification of functioning, disability and health. Geneva, Switzerland: World Health Organization;2001. p. 1–25.
3. Santucci C, Carioli G, Bertuccio P, Malvezzi M, Pastorino U, Boffetta P, et al. Progress in cancer mortality, incidence, and survival: a global overview. Eur J Cancer Prev. 2020; 29:367–81.

4. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.

5. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.

6. Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005; 53:2145–52.

7. Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017; 77:1009–20.
8. Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013; 63:295–317.

9. Stout NL, Binkley JM, Schmitz KH, Andrews K, Hayes SC, Campbell KL, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012; 118(8 Suppl):2191–200.

10. Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. 2017; 28:49–64.
11. Le Roy B, Pereira B, Bouteloup C, Costes F, Richard R, Selvy M, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial-the PREHAB study. BMJ Open. 2016; 6:e012876.

12. Tjernstrom F, Fransson PA, Kahlon B, Karlberg M, Lindberg S, Siesjo P, et al. Vestibular PREHAB and gentamicin before schwannoma surgery may improve long-term postural function. J Neurol Neurosurg Psychiatry. 2009; 80:1254–60.

13. Huang GH, Ismail H, Murnane A, Kim P, Riedel B. Structured exercise program prior to major cancer surgery improves cardiopulmonary fitness: a retrospective cohort study. Support Care Cancer. 2016; 24:2277–85.

14. Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer rehabilitation: an overview of current need, delivery models, and levels of care. Phys Med Rehabil Clin N Am. 2017; 28:1–17.
16. Wang XS, Cleeland CS, Mendoza TR, Engstrom MC, Liu S, Xu G, et al. The effects of pain severity on healthrelated quality of life: a study of Chinese cancer patients. Cancer. 1999; 86:1848–55.

17. Cella DF. Quality of life outcomes: measurement and validation. Oncology (Williston Park). 1996; 10(11 Suppl):233–46.
18. Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005; 14:464–77.

19. Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000; 5:353–60.

20. Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K. Management of cancer- related fatigue. Cancer Invest. 2005; 23:229–39.
21. Centeno C, Sanz A, Bruera E. Delirium in advanced cancer patients. Palliat Med. 2004; 18:184–94.

22. Gagnon P, Allard P, Masse B, DeSerres M. Delirium in terminal cancer: a prospective study using daily screening, early diagnosis, and continuous monitoring. J Pain Symptom Manage. 2000; 19:412–26.
23. Fann JR, Roth-Roemer S, Burington BE, Katon WJ, Syrjala KL. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer. 2002; 95:1971–81.

24. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005; 23:6712–8.

25. Braun IM, Rao SR, Pirl WF. Comparison of self-reported cognitive difficulties in a national sample of longterm cancer survivors and cancer-naïve controls. Psychosomatics. 2012; 53:68–74.

26. McDaniel JS, Musselman DL, Porter MR, Reed DA, Nemeroff CB. Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry. 1995; 52:89–99.
27. Alter CL, Pelcovitz D, Axelrod A, Goldenberg B, Harris H, Meyers B, et al. Identification of PTSD in cancer survivors. Psychosomatics. 1996; 37:137–43.

28. O’Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998; 79:1530–4.

29. Kirshblum S, O’Dell MW, Ho C, Barr K. Rehabilitation of persons with central nervous system tumors. Cancer. 2001; 92(4 Suppl):1029–38.

30. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004; 42:235–9.

31. Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978; 3:40–51.

32. Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin- Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004; 114:1362–7.

33. Healey JH, Brown HK. Complications of bone metastases: surgical management. Cancer. 2000; 88(12 Suppl):2940–51.
34. Bunting RW, Shea B. Bone metastasis and rehabilita tion. Cancer. 2001; 92(4 Suppl):1020–8.
35. Bowman C, Piedalue KA, Baydoun M, Carlson LE. The quality of life and psychosocial implications of cancer- related lower-extremity lymphedema: a systematic review of the literature. J Clin Med. 2020; 9:3200.

36. Ramos SM, O’Donnell LS, Knight G. Edema volume, not timing, is the key to success in lymphedema treatment. Am J Surg. 1999; 178:311–5.

37. Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis- Grant L, Smith R, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010; 304:2699–705.

38. Stubblefield MD. Clinical evaluation and management of radiation fibrosis syndrome. Phys Med Rehabil Clin N Am. 2017; 28:89–100.

39. Okunieff P, Augustine E, Hicks JE, Cornelison TL, Altemus RM, Naydich BG, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004; 22:2207–13.

40. Gillis TA, Donovan ES. Rehabilitation following bone marrow transplantation. Cancer. 2001; 92(4 Suppl):998–1007.

41. Jonker-Pool G, van Basten JP, Hoekstra HJ, van Driel MF, Sleijfer DT, Koops HS, et al. Sexual functioning after treatment for testicular cancer: comparison of treatment modalities. Cancer. 1997; 80:454–64.

42. Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer. 2002; 95:1773–85.

43. Siegel K, Raveis VH, Houts P, Mor V. Caregiver burden and unmet patient needs. Cancer. 1991; 68:1131–40.

44. Dumont S, Turgeon J, Allard P, Gagnon P, Charbonneau C, Vezina L. Caring for a loved one with advanced cancer: determinants of psychological distress in family caregivers. J Palliat Med. 2006; 9:912–21.

45. Lee WT, Chan HF, Wong E. Improvement of feeding independence in end-stage cancer patients under palliative care: a prospective, uncontrolled study. Support Care Cancer. 2005; 13:1051–6.

46. Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002; 34:1863–7.

47. Holtzman J, Schmitz K, Babes G, Kane RL, Duval S, Wilt TJ, et al. Effectiveness of behavioral interventions to modify physical activity behaviors in general populations and cancer patients and survivors. Evid Rep Technol Assess (Summ). 2004; (102):1–8.
48. Dimeo FC, Thomas F, Raabe-Menssen C, Propper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery. A randomised controlled trial. Support Care Cancer. 2004; 12:774–9.

49. Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005; 23:2378–88.

50. Redinbaugh EM, Baum A, Tarbell S, Arnold R. Endof- life caregiving: what helps family caregivers cope? J Palliat Med. 2003; 6:901–9.
51. Chen ML, Chu L, Chen HC. Impact of cancer patients’ quality of life on that of spouse caregivers. Support Care Cancer. 2004; 12:469–75.

52. Short PF, Vargo MM. Responding to employment concerns of cancer survivors. J Clin Oncol. 2006; 24:5138–41.

53. Spelten ER, Verbeek JH, Uitterhoeve AL, Ansink AC, van der Lelie J, de Reijke TM, et al. Cancer, fatigue and the return of patients to work-a prospective cohort study. Eur J Cancer. 2003; 39:1562–7.

54. Torok S, Kokonyei G, Karolyi L, Ittzes A, Tomcsanyi T. Outcome effectiveness of therapeutic recreation camping program for adolescents living with cancer and diabetes. J Adolesc Health. 2006; 39:445–7.

55. Mansky P, Sannes T, Wallerstedt D, Ge A, Ryan M, Johnson LL, et al. Tai chi chuan: mind-body practice or exercise intervention? Studying the benefit for cancer survivors. Integr Cancer Ther. 2006; 5:192–201.

56. Galski T, Williams JB, Ehle HT. Effects of opioids on driving ability. J Pain Symptom Manage. 2000; 19:200–8.

57. Lewis R, Patel B, Engle J. Cancer rehabilitation. In Gonzalez-Fernandez M, Schaaf S, editors. Handbook of physical medicine and rehabilitation. New York, NY: Springer Publishing Company;2022. p. 19–28.
Table 1.
Phases of cancer rehabilitation
Table 2.
Contraindications to exercise in patients with cancer




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download