Abstract
Notes
Ethics Statement
This case report was approved by the Institutional Review Board (British Hospital Institutional Bioethics Committee) and conducted according to the criteria set by the declaration of Helsinki. Written informed consent for publication of her details was obtained from the patient.
Author Contribution
Concept and design: FCZ, CMP, SG, and PB. Analysis and interpretation: FMG, MBB, MF, and MS. Data collection: FMG, MBB, FL, MF, and MS. Writing the article: FCZ, CMP, SG, and PB. Critical revision of the article: SG, CR, RR, and MFP. Final approval of the article: CR, RR, MFP, and PB. Statistical analysis: FL and MF.
REFERENCES
Fig. 1.
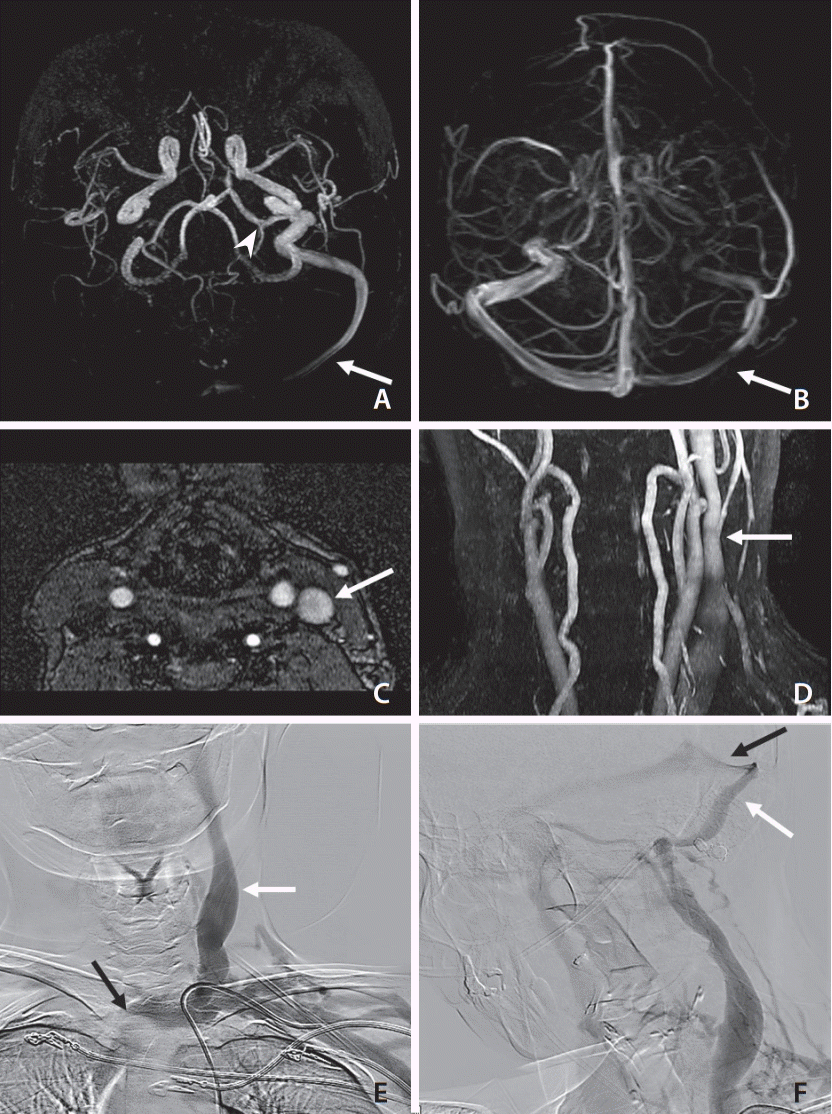
Table 1.
| Study | Sex/age (y) | Length of HD (y) | Previous CVC | CVC location | Previous renal transplant | AV shunt type/limb | Shunt usage time | Neurological manifestations | Central venous disease | Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lal et al. (1986) [10] | M/62 | 3 | Yes | Right SCV | No | AVF/right upper limb | 1 year | Decreased visual acuity, diplopia, retro-ocular throbbing headache, transient amaurosis | Right BCV stenosis | AVF ligation | Complete resolution of symptoms in 6 weeks |
| Molina et al. (1998) [11] | M/74 | 5 | Yes | Right and left SCV, right IJV | No | AVF and AVG/bilateral | 6 months | Decreased visual acuity, headache, blurry vision | Bilateral BCV stenosis | AVF ligation | Complete resolution of symptoms |
| Varelas et al. (1999) [12] | F/58 | NR | Yes | Right SCV | No | AVF/right upper limb | NR | Diplopia, right hemicra- nial headache, bilateral sixth nerve palsy | Right BCV stenosis | Angioplasty+stent placement | Resolution of ophthal- moplegia in 24 hours |
| Hartman et al. (2001) [13] | F/59 | 8 | NR | NR | Yes | AVF/left upper limb | 5 years | Headache, gait distur- bance, memory loss | Left BCV stenosis | AVF ligation | Resolution of hydro- cephalus and symp- toms in one week |
| Chang et al. (2004) [14] | F/50 | 3 | NR | NR | No | AVF/left upper limb | 3 years | Intermittent headache, retro-ocular pressure | Left BCV stenosis | Balloon angioplasty | Resolution of symp- toms and papilledema in 3 months |
| Cuadra et al. (2005) [15] | F/57 | NR | Yes | NR | Yes | AVG/right upper limb | NR | Headache, blurry vision | Right IJV, SCV, and axillary vein stenosis | AVG occlusion | Visual acuity was not recovered in the left eye |
| Nishimoto et al. (2005) [7] | F/62 | 9 | NR | NR | No | AVF/left upper limb | 9 years | Headache, seizures | Left BCV thrombosis | AVF ligation | Immediate resolution |
| Cleper et al. (2007) [5] | F/13 | 10.5 | NR | NR | Yes | AVF/left upper limb | 2 months | Right amaurosis, seizures | Right BCV and SCV occlusion | AVF ligation and creation of new access failed | The patient died |
| Watson and Russo (2007) [16] | F/36 | NR | NR | NR | No | AVF/left upper limb | NR | Headache, blurry vision | Left BCV occlusion | Recanalization of the left BCV | Complete resolution of symptoms |
| Nishijima et al. (2011) [17] | F/47 | 5 | NR | NR | No | AVF/left upper limb | 5 years | Right hemiplegia, headache, seizures | Left BCV occlusion | AVF ligation | Dramatic recovery from motor deficit |
| Saha et al. (2012) [18] | F/53 | 3 | NR | NR | No | AVG/ left upper limb | NR | Headache, lethargy | Left IJV stenosis | AVG occlusion | Complete resolution of symptoms |
| Samaniego et al. (2013) [6] | M/50 | 11 | NR | NR | No | AVG/right upper limb | 2 weeks | Headache, left homonymous hemianopia, encephalopathy | Left BCV occlusion | AVG occlusion | Full recovery one week later |
| Herzig et al. (2013) [3] | M/73 | NR | NR | NR | No | AVF/left upper limb | NR | Headache, blurry vision, seizures | Left BCV thrombosis | AVF ligation | Full recovery 2 days later |
| F/67 | NR | NR | NR | No | NR | NR | Right arm monoparesis, Involuntary movements of the right arm | Left BCV stenosis | Angioplasty+stent placement. Recanalization of the stent | Incomplete recovery with recurrence at seven months. | |
| Complete recovery four months after the second intervention | |||||||||||
| Salama et al. (2014) [4] | F/40 | NR | NR | NR | No | AVF/left upper limb | NR | Tinnitus, proptosis of the left eye | Left BCV occlusion | Angioplasty+stent placement | Recovery of symptoms in 24 hours |
| Prasad et al. (2015) [2] | M/47 | NR | NR | NR | No | AVG/left upper limb | NR | Right hemiparesis, altered mental status | Left BCV occlusion | Angioplasty+stent placement | Recovery of symptoms in the following days |
| Simon et al. (2014) [19] | M/65 | NR | Yes | Right IJV | No | AVF/Bilateral | NR | Decreased visual acuity, headache, blurry vision, tinnitus | Right BCV thrombosis | Angioplasty | Headache recovery in 24 hours. Visual acuity improved at 5 months |
| Mackay and Biousse (2015) [9] | F/60 | NR | NR | NR | No | AVF/right upper limb | AVF NR | Headache, blurry vision | Right SCV stenosis | Withdrawal of AVG. | Complete resolution of symptoms in 4 weeks |
| AVG/left upper limb | AVG 3 days | Ventriculoperitoneal shunt | |||||||||
| Kim et al. (2018) [8] | F/71 | 7 | NR | NR | No | AVG/ left upper limb | 7 years | Throbbing headache | Left BCV occlusion | Balloon angioplasty | Complete resolution of symptoms |
| F/63 | 10 | NR | NR | No | AVG/ left upper limb | 10 years | Seizures | Left BCV occlusion | Delayed treatment for sepsis | The patient died | |
| Haruma et al. (2020) [1] | M/53 | 4 | NR | NR | No | AVF/left upper limb | 4 years | Right hemiparesis, altered mental status, seizures | Left BCV stenosis | Angioplasty+stent placement | Symptom improvement without recurrence of stenosis |
| Iguchi et al. (2020) [20] | F/73 | 14 | NR | NR | Yes | AVG/ left upper limb | NR | Aphasia | Left BCV stenosis | AVG occlusion | Complete resolution of symptoms in one month |
| Caiza-Zambrano et al. (current study) | F/43 | 8 | Yes | Right IJV | Yes | AVF/left upper limb | 5 years | Abnormal right hand posture, retro-ocular headache, paresthesia | Incomplete left BCV thrombosis | OAC | Complete resolution of symptoms in three months |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download