Abstract
Abusive head trauma (AHT) is the most common and serious form of child abuse and a leading cause of traumatic death in infants and young children. The biomechanics of head injuries include violent shaking, blunt impact, or a combination of both. Neuroimaging plays an important role in recognizing and distinguishing abusive injuries from lesions from accidental trauma or other causes, because clinical presentation and medical history are often nonspecific and ambiguous in this age group. Understanding common imaging features of AHT can increase recognition with high specificity for AHT. In this review, we discuss the biomechanics of AHT, imaging features of AHT, and other conditions that mimic AHT.
In 1946, John Caffey, the most eminent of the pioneers of pediatric radiology, described a series of six cases of unexplained chronic subdural hematoma (SDH) and long-bone fractures in infants, and this observation led to the formation of a unique medical entity of child abuse [5]. The term “battered child syndrome” was coined by Kempe et al. [35] in 1962 and the authors suggested that the syndrome should be considered in any child with a combination of multiple fractures, SDH, and bruises. In 1974, Caffey [7] coined the term “whiplash shaken infant syndrome” to describe children suffering from SDH, retinal hemorrhages, and neurologic injury. The terms “battered child syndrome,” “parent-infant traumatic stress syndrome,” and “shaken baby syndrome” have all been used; however, in 2009, the American Academy of Pediatrics Committee on Child abuse and Neglect recommended that the term “abusive head trauma (AHT)” should be used because it more appropriately described traumatic injuries to the brain resulting from abuse [15]. While the term “shaken baby syndrome” implies a single mechanism of head trauma, AHT further encompasses multiple abusive mechanisms, such as crushing head injury, shaking, shaking with impact, impact alone, and strangulation.
AHT accounts for 80% of non-accidental trauma deaths and remains a leading cause of death in infants and young children [10,50]. Adverse neurodevelopmental outcomes are common among survivors of AHT [10,25,29,53]. AHT is a global issue that requires regional and global sharing of clinical and biomechanical data to increase the efficiency and speed of developing and implementing prevention, detection, and treatment programs for AHT in children. In South Korea, the number of child abuse reports is increasing every year and the number of child abuse reports reached approximately 42000 cases in 2020 [38].
Imaging plays a crucial role in the evaluation of children with suspected AHT and aids in accurate diagnosis, as clinical presentation may be non-specific. This review investigates the central nervous system (CNS) injuries that characterize AHT, with a focus on pathophysiology and imaging appearance. Readers are encouraged to explore the various imaging features of these AHTs.
The biomechanics of AHT may include both impact loading and impulsive loading. The “impact loading” refers to the application of direct forces to the head, whereas “impulsive loading” refers to non-impact forces produced by alternating angular acceleration and deceleration of the cranial vault (i.e., shaking injury, Fig. 1). With inflicted injury, the brain moves more than fixed structure such as the falx cerebri, tentorium cerebelli, dural venous sinuses, and bony eminence. The shearing forces created may cause a spectrum of injury, including SDH, subarachnoid hemorrhage, contusion, diffuse axonal injury, and parenchymal laceration. In previous studies, authors described the association of a shaking-type injury with SDH, retinal hemorrhage, and focal or diffuse parenchymal injury, referred as the “triad” of AHT [6,8] (Figs. 2 and 3). Impact loading typically results in skull fracture and parenchymal contusions with associated focal extra-axial and subperiosteal hemorrhage [9] (Figs. 4-7). Impact injuries are less common in infants with AHT, but occur more frequently in older children [2,31]. Short-distance falls, such as from a couch or standing height, are common in infants and young children and usually result in minimal injury [45,48]. However, a reported short-distance fall is a common history provided by caretakers of infants who are subsequently determined to have been abused [3,19,47,54]. Therefore, serious injury from a reported short-distance fall should always prompt concern for abuse.
Plain radiographs have a limited role in detecting head injury, although they are helpful in detecting fracture and are performed as part of a skeletal survey in children with suspected abusive injuries.
Computed tomography (CT) is the most sensitive imaging modality for detecting skull fractures and acute intracranial hemorrhage. Non-contrast-enhanced CT has been used as an initial screening test for suspected AHT. Volume-rendered CT images (Fig. 6C) and multiplanar reformatting (Fig. 4C and D) can increase the conspicuity of subtle fracture lines and minor extra-axial hemorrhage [19,54]. Although CT has a risk of radiation exposure, the benefit of promptly identifying skull fractures, subtle intracranial hemorrhage, and potential life-threatening cerebral edema usually outweighs these risks.
Brain magnetic resonance imaging (MRI) should be performed in any child with an abnormal head CT, persistent neurologic signs or symptoms, and a high index of suspicion for abuse [30,44,48]. Unenhanced MRI with diffusion weighted imaging (DWI), gradient echo sequence or susceptibility-weighted imaging (SWI) is sensitive in detecting parenchymal injury and small amounts of hemorrhage. It can also provide valuable prognostic information [46,56]. Gadolinium-based contrast material is not routinely administered, although it may increase the accuracy of dating extra-axial collections in some patients [41,54].
Intracranial injury may occur in asymptomatic patients with extracranial injuries suspected for non-accidental trauma [4,16,17,40]. Routine screening of the CNS in all children with sufficient clinical concern of child abuse is therefore recommended by many authorities.
Unsuspected spinal injuries develop in approximately 75% of patients with AHT according to some estimates [11,30,42]. MRI of the cervical spine should routinely be performed at the time of brain imaging to assess bone, ligament, and spinal cord injury. Whole-spine MRI aids in detecting spinal SDH in the setting of spinal injuries during trauma investigation or abnormal findings on a neurologic examination [33,42].
Skeletal injuries commonly occur with AHT in infants and young children. Although certain patterns of intracranial injury may suggest child abuse, they are commonly not diagnostic. Therefore, if suspected AHT is accompanied by unsuspected skeletal injuries, it has important implications, particularly when a highly specific fracture, such as metaphyseal corner fracture, is present. All infants and young children with suspected AHT should undergo a radiographic skeletal survey according to American College of Radiology (ACR) guidelines [17,34]. In most cases, follow-up skeletal examination is required after 2 weeks. Focused MRI is especially valuable in cases of epiphyseal separation and vertebral fractures or dislocations.
Isolated linear skull fractures (Fig. 5C) are low in specificity for AHT. Linear skull fractures and small associated extra-axial or subperiosteal hemorrhage (Fig. 5) are common findings in low-impact accidental head trauma. However, multiple (Figs. 4 and 7), complex, diastatic, or growing skull fractures (Fig. 6) suggest a high-energy mechanism and are associated with AHT without an appropriate history of trauma. Nevertheless, an isolated skull fracture in children without a proven incidental cause of injury should prompt a skeletal survey to rule out additional injury.
CT performed in the bone algorithm reconstructed with submillimeter slice thickness (Fig. 4D) is the best modality for evaluating skull fractures in children. When skull fractures occur, multi-planar reconstructions and three-dimensional reformatting of the skull help to differentiate the accessory sutures and wormian bones from subtle fractures. Dating of skull fractures on CT is very difficult unless coexisting superficial soft tissue swelling or intracranial abnormalities are observed.
SDH is the most common neuroradiological finding of AHT [24,26]. It is commonly attributed to the rupture of fragile veins crossing the subdural space secondary to shear forces, typically due to shaking injury [52] (Fig. 3A-C). Other potential causes of SDH are injury to the dura itself. Extra-axial fluid collections often occur in AHT, and information regarding the age of these fluid accumulations are usually beneficial to physicians. However, the dating of extra-axial hemorrhage is not always possible [45,51]. Dating these collections with CT attenuation values is limited and should be evaluated by radiologists with caution. The presence of high-attenuated extra-axial fluid collection is consistent with acute hematoma (Figs. 3A, 4C, 5D, 6E); however, homogeneously low-attenuated fluid collection can be manifested acutely by arachnoid shearing (Fig. 3D). Due to these confounding variables, dating of extraaxial hemorrhage using CT has to be reserved if previous images are available for reference.
Birth-related SDHs are typically posteriorly located [36,43] (Fig. 8). Small clinically insignificant SDHs have been reported in 26–62% of newborns and occur following both vaginal and caesarian deliveries. Most of them resolved within the first month of life, and asymptomatic SDH related to birth was found to be developmentally normal at 24 months of age [36,43]. In contrast, SDH related to AHT, has a strong association with parenchymal edema (Figs. 2A, 4C, E, 7B-E) and restricted diffusion (Figs. 3F and 4F), and often progresses to severe encephalomalacia (Figs. 2B, C, 4G) [54]. Unexplained SDH in infants should always prompt concerns about AHT.
The nature of the parenchymal injury seen in AHT by impulse loading is not well understood. Although parenchymal injury was initially assumed to reflect traumatic diffuse axonal injury, several more recent studies have shown that the predominant parenchymal injury in children with AHT is likely hypoxic-ischemic injury (Figs. 2, 4, 7) rather than traumatic diffuse axonal injury [22,23]. Diffuse parenchymal injury is not specific for AHT; however, when it is associated with SDH, retinal hemorrhage, or cervicomedullary injury, it is highly suggestive of AHT [14,30,32]. AHT-related diffuse parenchymal injury may not be apparent at early CT screening (Fig. 3D) or may manifest as hemispheric or diffuse hypoattenuation with mass effect (Figs. 2A, 4C, 7B, C). Early parenchymal injury is better visualized on MRI. DWI dramatically increases the conspicuity of cytotoxic injury (Figs. 3F and 4F) because the T2 prolongation of brain edema is subtle in the unmyelinated brain [55]. Most AHT cases have shown a watershed pattern of diffusion restriction (Fig. 4F) [28,55]; however, multiple patterns of AHT-related parenchymal injury can be seen.
Focal parenchymal injury may result from either impulsive- or impact-loading injury mechanisms in AHT and is less common and lesser characterized than diffuse parenchymal injury. Parenchymal lacerations (Fig. 6D), also known as contusional tears, appear as linear disruptions of the brain parenchyma predominately within the subcortical white matter or at the gray-white junction of the gyrus. They predominate in the frontal lobes. They are commonly hemorrhagic with layering sedimentation levels on both CT and MRI and may be associated with overlying subpial hemorrhage. Focal parenchymal lesions may rarely occur in older children and are presumed to be associated with traumatic diffuse axonal injury. They are characterized by multiple foci of T2 hyperintensity that are most commonly seen in the frontotemporal white matter, corpus callosum, and cerebral peduncles.
Non-traumatic dural sinus thrombosis has been suggested as a cause of findings seen in AHT. A review of 45 children diagnosed with AHT found evidence of direct trauma to the bridging veins in 44% of patients, with SDH in 91% of them and retinal hemorrhages in 71% of them [12]. However, non-traumatic venous thrombosis is rare and often associated with predisposing conditions, such as dehydration, malignancy, thrombosis, and sepsis. McLean et al. [37] studied 36 children with non-traumatic intracranial venous thrombosis, and none of them had SDH; of these, 13 had an infectious cause, six had dehydration, five had leukemia, and four had iron deficiency anemia [37].
Retinal hemorrhages are estimated to occur in approximately 85% of infants and young children with AHT. However, as with SDHs, retinal hemorrhages have only moderate specificity for AHT [21,49]. AHT is more predictable when retinal hemorrhages are bilateral, multilayered (pre-, intra-, and subretinal), and peripherally extend to the ora serrata at funduscopy. Retinal hemorrhages have been occasionally reported in imaging but are yet to be traditionally considered an imaging diagnosis [1]. They appear as punctate high-density foci in the posterior globes on CT images with corresponding low-signal-intensity foci on magnetic resonance images because of susceptibility artifacts (Fig. 3G). Gradient echo or SWI has increased the sensitivity of MRI for the detection of retinal hemorrhages. A dedicated high-resolution orbital SWI sequence increased the sensitivity to 80% [56]. Retinal hemorrhages identified at MRI should prompt concerns of AHT, because an increasing severity of retinal hemorrhage is correlated with an increasing risk for AHT. In the absence of a history of severe trauma, detection of a signal-intensity abnormality in the posterior globes in children should prompt evaluation of additional intracranial injuries and clinical consideration of dilated funduscopy.
The incidence of spinal cord injury in AHT cases was found in only 3.2% of all children with spine injury. Injuries were predominantly located in the cervical spine (73%) with a predominance of the upper cervical spine (88%). In AHT, the whiplash-shaking injury mechanism plays an important role in brainstem and cervical spine injuries. Autopsy results showed cervical spinal cord contusions in five of six (83%) children who were exposed to AHT [22,23]. Focal axonal damage to the brainstem and spinal nerve roots were found in a neuropathological study in 11 of 37 (30%) AHT cases, but none in controls. The presence of spinal injury indicates a severe form of AHT with high risk, long-term neurological impairment [27,30]. The radiological findings of cervical spine injury in AHT can be subtle. Detection of cervical spine injuries by MRI does not discriminate between accidental injury and AHT. Kadom et al. [30] described hyperintensity of the spinal cord on T2-weighted image with extensive brain injuries and ligamentous injuries in two infants with AHT.
Cervical spine MRI should be included in the imaging evaluation of all infants with suspected AHT. The most commonly reported finding in this region is ligamentous injury [54]. Fat-saturated sequences are especially helpful. Cervical ligamentous injuries were identified in a significantly greater proportion in AHT cases (78%) than in accidental injury cases (46%) [13].
Benign enlarged subarachnoid spaces (BESS) commonly occur in children who are less than 1 year of age, and an association [20] has been reported between SDH and BESS. An isolated SDH in the setting of known BESS may be due to AHT [39]. In cases of potential AHT, MRI can be helpful to determine whether a subdural collection contains hemorrhagic products (Fig. 5A and B). An additional confounding intracranial pathology is glutaric aciduria type 1 [18], a condition associated with brain atrophy and subdural fluid collections (Fig. 9). It is a rare metabolic disorder, occurring in one out of 30000 births and can be diagnosed through advanced serologic testing. Menkes Syndrome (Fig. 10), also a rare metabolic disorder involving copper accumulation that can be identified through genetic studies and hair analysis, may generate findings that overlap with non-accidental trauma [18].
Biomechanisms of AHT include both impact loading and impulsive loading. Non-contrast-enhanced CT is the best screening tool for suspected AHT. In addition, MRI, including DWI and hemorrhage-sensitive sequences can provide more detailed information concerning parenchymal injury, hemorrhage, and even retinal hemorrhage. Skull fractures and SDHs are common findings of AHT, and diverse patterns of fractures may be seen according to the mechanisms of the injury. Skull fracture or SDH without a proven incidental cause should prompt a skeletal survey. Parenchymal injury patterns can reflect diffuse hypoxic-ischemic injury, traumatic diffuse axonal injury, or focal contusional tear. AHT-associated spinal cord lesions or cervical ligamentous injuries can be revealed by cervical spine MRI. In imaging interpretation, the presence of BESS, birth trauma, and several metabolic encephalopathies with SDH, should also be considered.
Notes
References
1. Altinok D, Saleem S, Zhang Z, Markman L, Smith W. MR imaging findings of retinal hemorrhage in a case of nonaccidental trauma. Pediatr Radiol. 39:290–292. 2009.

2. Bechtel K, Stoessel K, Leventhal JM, Ogle E, Teague B, Lavietes S, et al. Characteristics that distinguish accidental from abusive injury in hospitalized young children with head trauma. Pediatrics. 114:165–168. 2004.

3. Bertocci GE, Pierce MC, Deemer E, Aguel F, Janosky JE, Vogeley E. Using test dummy experiments to investigate pediatric injury risk in simulated short-distance falls. Arch Pediatr Adolesc Med. 157:480–486. 2003.

4. Boehnke M, Mirsky D, Stence N, Stanley RM, Lindberg DM, ExSTRA investigators. Occult head injury is common in children with concern for physical abuse. Pediatr Radiol. 48:1123–1129. 2018.

5. Caffey J. Multiple fractures in the long bones of infants suffering from chronic subdural hematoma. Am J Roentgenol Radium Ther. 56:163–173. 1946.
6. Caffey J. On the theory and practice of shaking infants. Its potential residual effects of permanent brain damage and mental retardation. Am J Dis Child. 124:161–169. 1972.
7. Caffey J. The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics. 54:396–403. 1974.

8. Case ME. Distinguishing accidental from inflicted head trauma at autopsy. Pediatr Radiol 44 Suppl. 4:S632–S640. 2014.

9. Case ME, Graham MA, Handy TC, Jentzen JM, Monteleone JA; National Association of Medical Examiners Ad Hoc Committee on Shaken Baby Syndrome. Position paper on fatal abusive head injuries in infants and young children. Am J Forensic Med Pathol. 22:112–122. 2001.

10. Chevignard MP, Lind K. Long-term outcome of abusive head trauma. Pediatr Radiol 44 Suppl. 4:S548–S558. 2014.

11. Choudhary AK, Bradford RK, Dias MS, Moore GJ, Boal DK. Spinal subdural hemorrhage in abusive head trauma: a retrospective study. Radiology. 262:216–223. 2012.

12. Choudhary AK, Bradford R, Dias MS, Thamburaj K, Boal DK. Venous injury in abusive head trauma. Pediatr Radiol. 45:1803–1813. 2015.

13. Choudhary AK, Ishak R, Zacharia TT, Dias MS. Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr Radiol. 44:1130–1140. 2014.

14. Choudhary AK, Servaes S, Slovis TL, Palusci VJ, Hedlund GL, Narang SK, et al. Consensus statement on abusive head trauma in infants and young children. Pediatr Radiol. 48:1048–1065. 2018.

15. Christian CW, Block R; Committee on Child Abuse and Neglect; American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics. 123:1409–1411. 2009.

16. Danaher F, Vandeven A, Blanchard A, Newton AW. Recognizing, diagnosing, and preventing child maltreatment: an update for pediatric clinicians. Curr Opin Pediatr. 30:582–590. 2018.
17. Expert Panel on Pediatric Imaging, Wootton-Gorges SL, Soares BP, Alazraki AL, Anupindi SA, Blount JP, et al. ACR appropriateness criteria® suspected physical abuse-child. J Am Coll Radiol. 14(5S):S338–S349. 2017.
18. Fernando S, Obaldo RE, Walsh IR, Lowe LH. Neuroimaging of nonaccidental head trauma: pitfalls and controversies. Pediatr Radiol. 38:827–838. 2008.

19. Flom L, Fromkin J, Panigrahy A, Tyler-Kabara E, Berger RP. Development of a screening MRI for infants at risk for abusive head trauma. Pediatr Radiol. 46:519–526. 2016.

20. Foerster BR, Petrou M, Lin D, Thurnher MM, Carlson MD, Strouse PJ, et al. Neuroimaging evaluation of non-accidental head trauma with correlation to clinical outcomes: a review of 57 cases. J Pediatr. 154:573–577. 2009.

21. Forbes BJ, Rubin SE, Margolin E, Levin AV. Evaluation and management of retinal hemorrhages in infants with and without abusive head trauma. J AAPOS. 14:267–273. 2010.

22. Geddes JF, Hackshaw AK, Vowles GH, Nickols CD, Whitwell HL. Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain. 124(Pt 7):1290–1298. 2001.

23. Geddes JF, Vowles GH, Hackshaw AK, Nickols CD, Scott IS, Whitwell HL. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain. 124(Pt 7):1299–1306. 2001.

24. Greeley CS. Abusive head trauma: a review of the evidence base. AJR Am J Roentgenol. 204:967–973. 2015.

25. Greiner MV, Lawrence AP, Horn P, Newmeyer AJ, Makoroff KL. Early clinical indicators of developmental outcome in abusive head trauma. Childs Nerv Syst. 28:889–896. 2012.

26. Gunda D, Cornwell BO, Dahmoush HM, Jazbeh S, Alleman AM. Pediatric central nervous system imaging of nonaccidental trauma: beyond subdural hematomas. Radiographics. 39:213–228. 2019.

27. Huisman TA, Wagner MW, Bosemani T, Tekes A, Poretti A. Pediatric spinal trauma. J Neuroimaging. 25:337–353. 2015.

28. Ichord RN, Naim M, Pollock AN, Nance ML, Margulies SS, Christian CW. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J Neurotrauma. 24:106–118. 2007.

29. Jackson JE, Beres AL, Theodorou CM, Ugiliweneza B, Boakye M, Nuño M. Long-term impact of abusive head trauma in young children: outcomes at 5 and 11 years old. J Pediatr Surg. 56:2318–2325. 2021.

30. Kadom N, Khademian Z, Vezina G, Shalaby-Rana E, Rice A, Hinds T. Usefulness of MRI detection of cervical spine and brain injuries in the evaluation of abusive head trauma. Pediatr Radiol. 44:839–848. 2014.

31. Kelly P, John S, Vincent AL, Reed P. Abusive head trauma and accidental head injury: a 20-year comparative study of referrals to a hospital child protection team. Arch Dis Child. 100:1123–1130. 2015.

32. Kemp AM, Jaspan T, Griffiths J, Stoodley N, Mann MK, Tempest V, et al. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 96:1103–1112. 2011.

33. Kemp AM, Joshi AH, Mann M, Tempest V, Liu A, Holden S, et al. What are the clinical and radiological characteristics of spinal injuries from physical abuse: a systematic review. Arch Dis Child. 95:355–360. 2010.

34. Kemp AM, Rajaram S, Mann M, Tempest V, Farewell D, Gawne-Cain ML, et al. What neuroimaging should be performed in children in whom inflicted brain injury (iBI) is suspected? A systematic review. Clin Radiol. 64:473–483. 2009.

35. Kempe CH, Silverman FN, Steele BF, Droegemueller W, Silver HK. The battered-child syndrome. JAMA. 181:17–24. 1962.

36. Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 242:535–541. 2007.

37. McLean LA, Frasier LD, Hedlund GL. Does intracranial venous thrombosis cause subdural hemorrhage in the pediatric population? AJNR Am J Neuroradiol. 33:1281–1284. 2012.

38. Ministry of Culture, Sports and Tourism. Child abuse & neglect Korea 2020. Available at : https://www.korea.kr/archive/expDocView.do?docId=39601.
39. Pfeifer CM, Hammer MR, Mangona KL, Booth TN. Non-accidental trauma: the role of radiology. Emerg Radiol. 24:207–213. 2017.

40. Pfeifer CM, Henry MK, Caré MM, Christian CW, Servaes S, Milla SS, et al. Debunking fringe beliefs in child abuse imaging: AJR expert panel narrative review. AJR Am J Roentgenol. 217:529–540. 2021.

41. Porto L, Bartels MB, Zwaschka J, You SJ, Polkowski C, Luetkens J, et al. Abusive head trauma: experience improves diagnosis. Neuroradiology. 63:417–430. 2021.

42. Rabbitt AL, Kelly TG, Yan K, Zhang J, Bretl DA, Quijano CV. Characteristics associated with spine injury on magnetic resonance imaging in children evaluated for abusive head trauma. Pediatr Radiol. 50:83–97. 2020.

43. Rooks VJ, Eaton JP, Ruess L, Petermann GW, Keck-Wherley J, Pedersen RC. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol. 29:1082–1089. 2008.

44. Sieswerda-Hoogendoorn T, Boos S, Spivack B, Bilo RA, van Rijn RR. Abusive head trauma part II: radiological aspects. Eur J Pediatr. 171:617–623. 2012.
45. Sieswerda-Hoogendoorn T, Postema FAM, Verbaan D, Majoie CB, van Rijn RR. Age determination of subdural hematomas with CT and MRI: a systematic review. Eur J Radiol. 83:1257–1268. 2014.

46. Thamburaj K, Soni A, Frasier LD, Tun KN, Weber SR, Dias MS. Susceptibility-weighted imaging of retinal hemorrhages in abusive head trauma. Pediatr Radiol. 49:210–216. 2019.

47. Thompson A, Bertocci G. Pediatric bed fall computer simulation model: parametric sensitivity analysis. Med Eng Phys. 36:110–118. 2014.

48. Vázquez E, Delgado I, Sánchez-Montañez A, Fábrega A, Cano N. Imaging abusive head trauma: why use both computed tomography and magnetic resonance imaging? Pediatr Radiol. 44 Suppl 4:S589–S603. 2014.

49. Wang L, Petrak M, Holz FG, Müller A, Krohne TU. Retinal hemorrhages in shaken baby syndrome. J Pediatr. 207:256. 2019.

50. Wilson TA, Gospodarev V, Hendrix S, Minasian T. Pediatric abusive head trauma: ThinkFirst national injury prevention foundation. Surg Neurol Int. 12:526. 2021.

51. Wittschieber D, Karger B, Niederstadt T, Pfeiffer H, Hahnemann ML. Subdural hygromas in abusive head trauma: pathogenesis, diagnosis, and forensic implications. AJNR Am J Neuroradiol. 36:432–439. 2015.

52. Wittschieber D, Karger B, Pfeiffer H, Hahnemann ML. Understanding subdural collections in pediatric abusive head trauma. AJNR Am J Neuroradiol. 40:388–395. 2019.

53. Wright J, Painter S, Kodagali SS, Jones NR, Roalfe A, Jayawant S, et al. Disability and visual outcomes following suspected abusive head trauma in children under 2 years. Arch Dis Child. 106:590–593. 2021.

Fig. 1.
Illustration explaining the mechanism of abusive head trauma by vigorous shaking of the baby. Rapid alternating angular acceleration and deceleration of the head results in subdural hemorrhage, parenchymal injury, and retinal hemorrhage, often without external signs of injury.
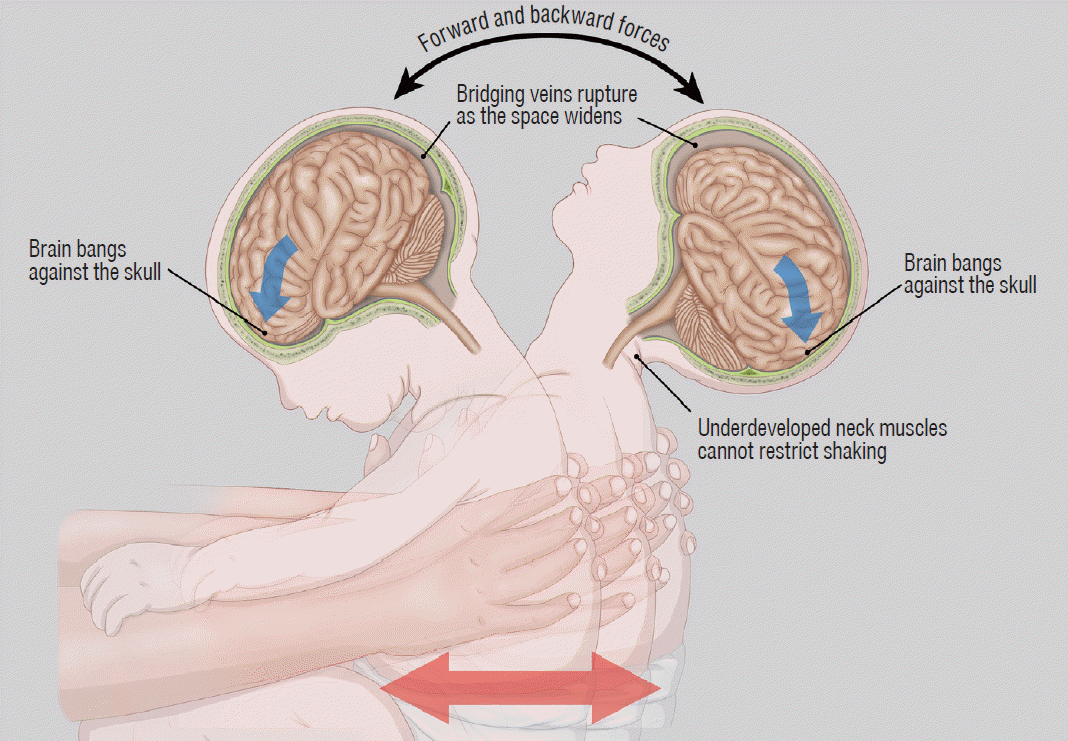
Fig. 2.
Three-month-old boy with seizure-like movements, multiple bruises, and retinal hemorrhage. A : Non-contrast-enhanced computed tomography image shows bilateral subdural fluid and ill-defined low attenuation of the cerebral parenchyma. B and C : Follow-up magnetic resonance images reveal an atrophic brain with multifocal tissue loss and increased amount of subdural hemorrhage (SDH) at both convexities, which shows higher signal intensity than that of the cerebrospinal fluid on both T1- (B) and T2- (C) weighted image. Separated compartments with fluid levels (arrows) containing blood products exhibiting different signal intensities are suggestive of multistage SDH.
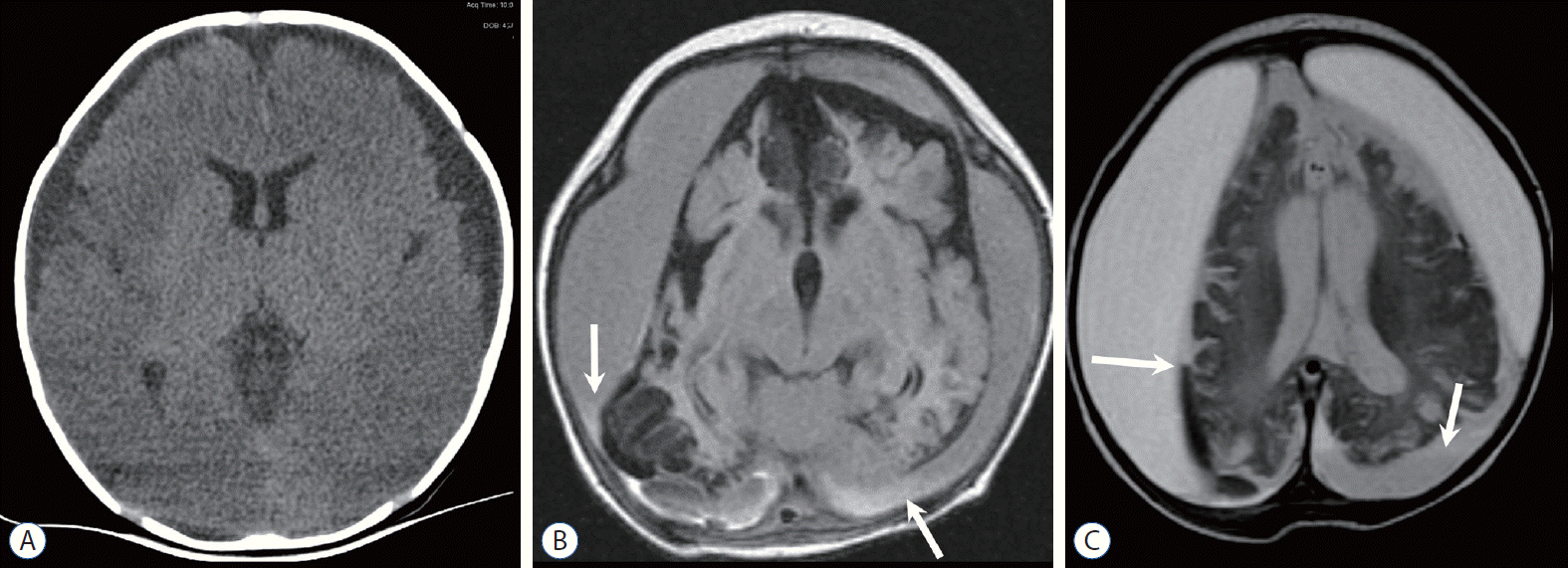
Fig. 3.
Four-month-old girl observed with lethargy and whole-body cyanosis in bed. A : Initial Non-contrast-enhanced computed tomography (NECT) at the level of the vertex shows multiple small high-density hemorrhages (arrows) at the gyral surface. B and C : Follow-up T2-weighted image (T2WI) (B) and gradient echo image (C) at the same level demonstrate multiple dark signal intensities (arrows) at the site of bridging vein rupture. Focal distension or blooming of the veins is suggestive of clots or thrombi. D : NECT obtained 2 days after the initial CT shows subdural hemorrhage posteriorly (arrows) and rapid onset bilateral subdural hygroma at the convexities. E : T2WI shows ill-defined increase in signal intensity of the cerebral hemispheres and splenium (arrow). F : Diffusion weighted image also shows increased signal intensity in the splenium (arrow), as well as both occipital lobes, and gray-white junction of the left frontal lobe. Diverse degrees of apparent diffusion coefficient (ADC) decline was demonstrated on an ADC map (not shown). G : Axial gradient echo images reveal tiny dark spots at the posterior wall of both eyes, indicating retinal hemorrhage (arrows). Hemorrhage is also noted in the right anterior chamber (black arrow). Although she had only a minor trauma history of being bumped into the door, skeletal survey revealed fracture of the clavicle, metacarpal bone, and spine, and eye examination disclosed fresh, multifocal, and multi-layered retinal hemorrhage.
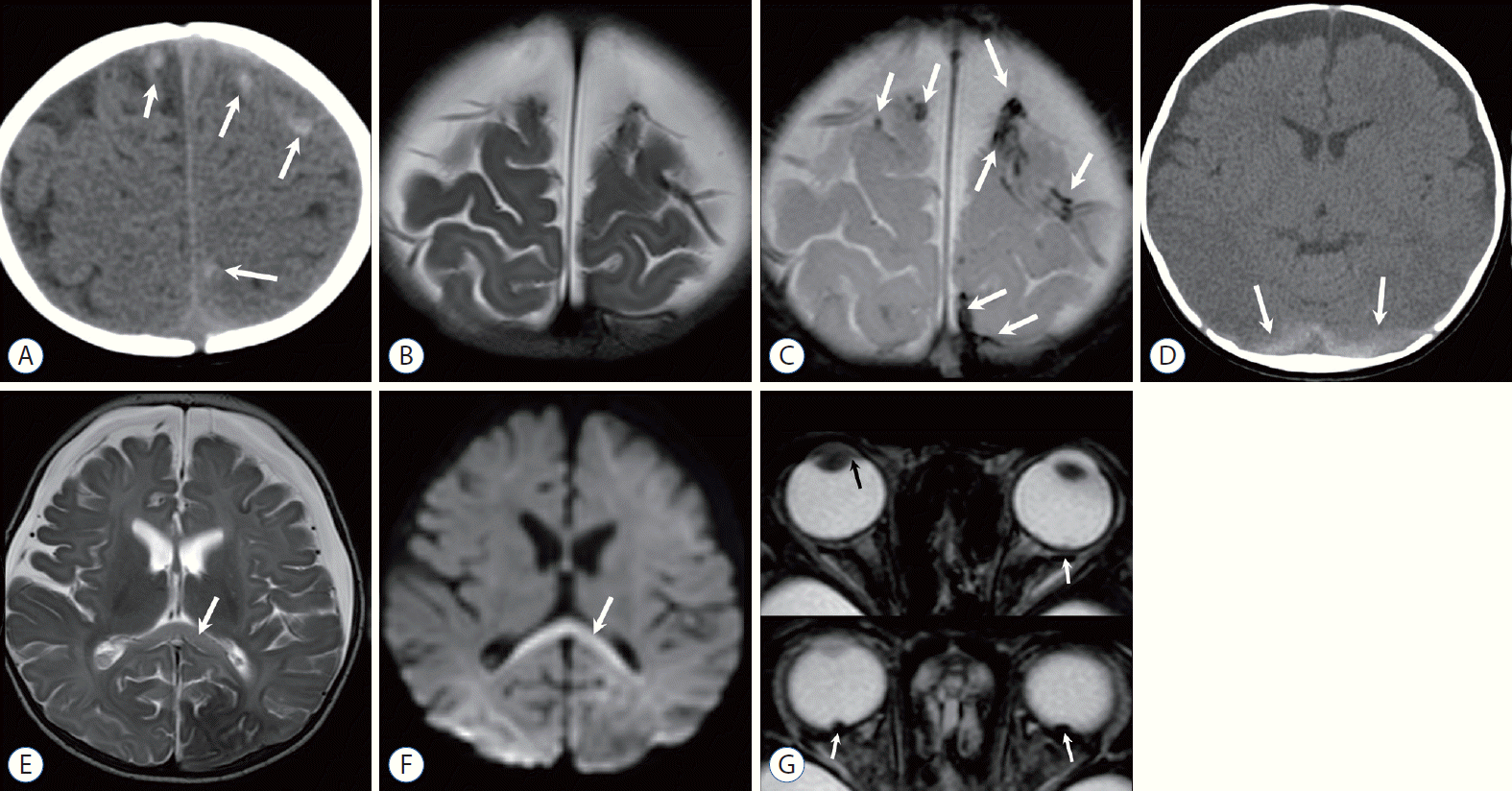
Fig. 4.
Two-month-old girl with a history of minor trauma. A and B : Anteroposterior (AP) and lateral skull radiographs demonstrate multiple linear fractures (arrows) of eggshell type. C : Non-contrast-enhanced computed tomography (NECT) coronal reconstructed image well demonstrates high density subdural hemorrhage adjacent to the falx and tentorium (arrows), as well as scalp swelling with hemorrhage. Ill-defined, decreased attenuation of the parenchyma suggests an acute edematous lesion. D : Bone window setting image clearly shows multifocal fracture and displaced fragments (arrows). E : Coronal T2-weighted image (T2WI) shows hemorrhagic contusion of the left temporal lobe (arrowhead), extra-parenchymal fluid or hemorrhage, and increased signal intensity at the parasagittal areas (arrows) suggest watershed injury. F : Diffusion restriction is mainly distributed in parasagittal areas and posterior thalami (arrows), although other parts of the brain are involved, on an apparent diffusion coefficient (ADC) map. G : The areas of ADC decline and T2 high intensity eventually evolved to a severe encephalomalacia on a follow-up T2WI.
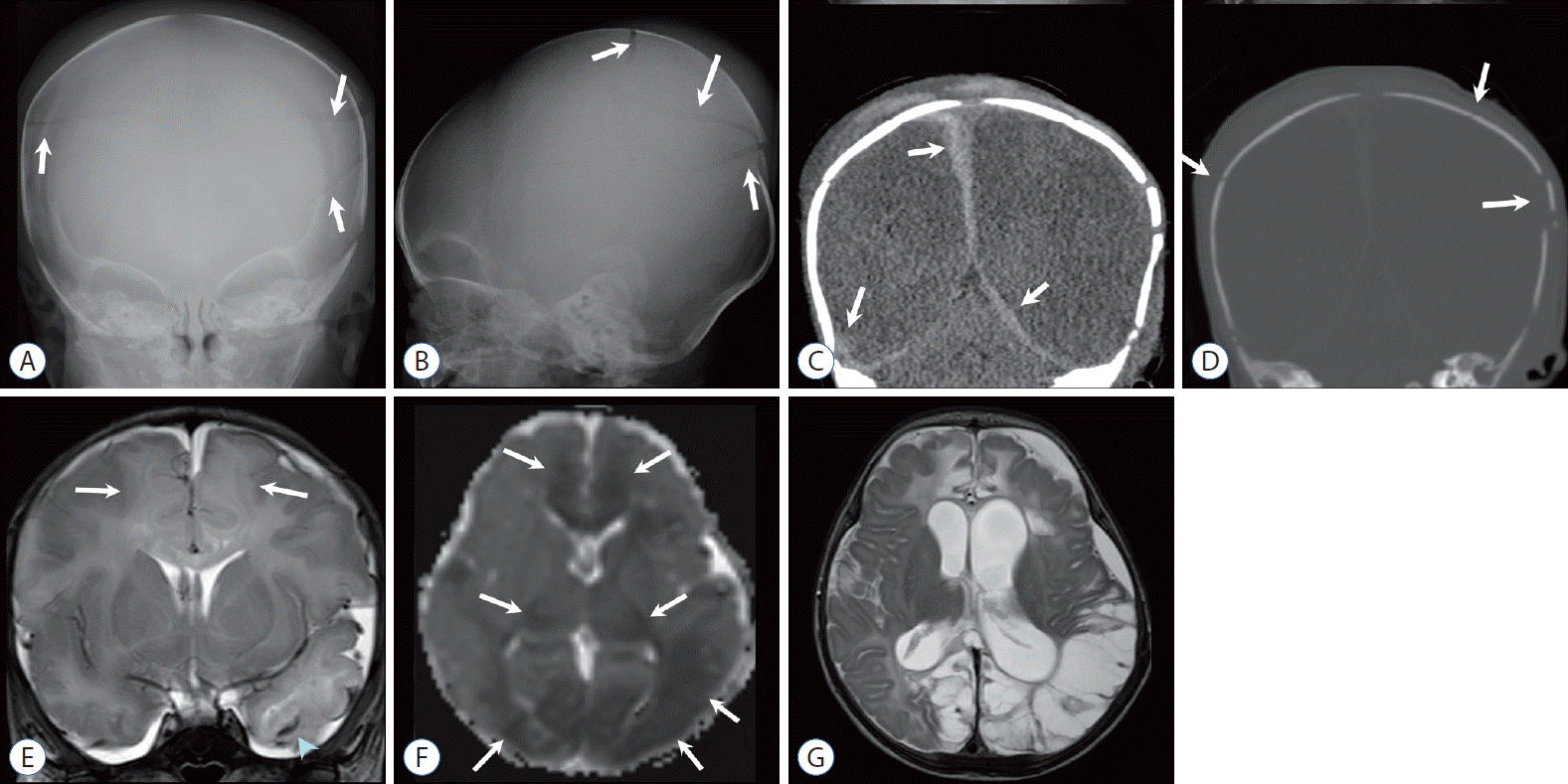
Fig. 5.
Five-month-old girl with incidentally detected subdural hemorrhage (SDH) during follow-up for retinoblastoma, and another episode of head trauma. A : Small amount of left frontal SDH (arrow) is incidentally noted on a coronal T1-weighted image (T1WI) of the orbit magnetic resonance imaging (MRI). B : Subsequent contrast-enhanced computed tomography for evaluation of abusive head trauma shows bilateral subarachnoid space widening where enhanced vessels are traversing. Note a localized cerebrospinal fluid space at the left frontal convexity (arrow) displacing a vascular structure, that corresponds to the subdural collection noted on a previous MRI (A). There is no visible skull fracture. C : Skull radiography, obtained after a week, when the baby was presented to the emergency room for irritability and scalp swelling, reveals linear fracture of the left parietal bone (arrow). D : Non-contrast-enhanced computed tomography shows SDH at the right convexity (arrow), while there is a hemorrhagic swelling of the left scalp adjacent to the left parietal fracture site. Eye examination under anesthesia revealed regressed calcified tumor and retinal hemorrhage.
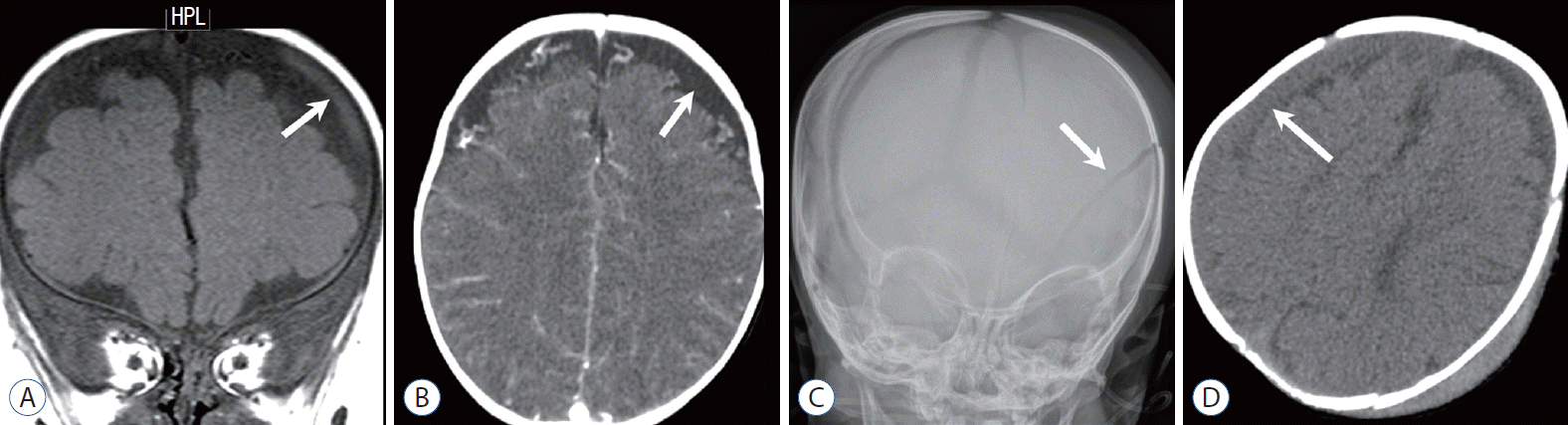
Fig. 6.
Three-month-old boy with a growing skull fracture and repeated head injury. A and B : Anteroposterior (AP) and lateral skull radiographs show a displaced fracture margin (arrows in A) and an enlarged defect (arrows in B). C : Volume-rendered computed tomography image demonstrates a complex defect caused by a displaced fracture margin (arrows), suggesting a “growing fracture.” D : Coronal T2-weighted image (T2WI) demonstrates a herniated pseudomeningocele or leptomeningeal cyst (arrow) via the enlarged fracture defect and focal parenchymal laceration (black arrow). E : Follow-up non-contrast-enhanced computed tomography obtained 2 weeks after reveals multifocal additional high-density hemorrhage in the protruded meningeal sac, parenchyma, and para-falcine areas (arrows) and parenchymal lesions of low attenuation (black arrow), suggesting repeated injury. Past medical records revealed repeated episodes of physical injury, and skeletal survey demonstrated multistage fracture of the bones.
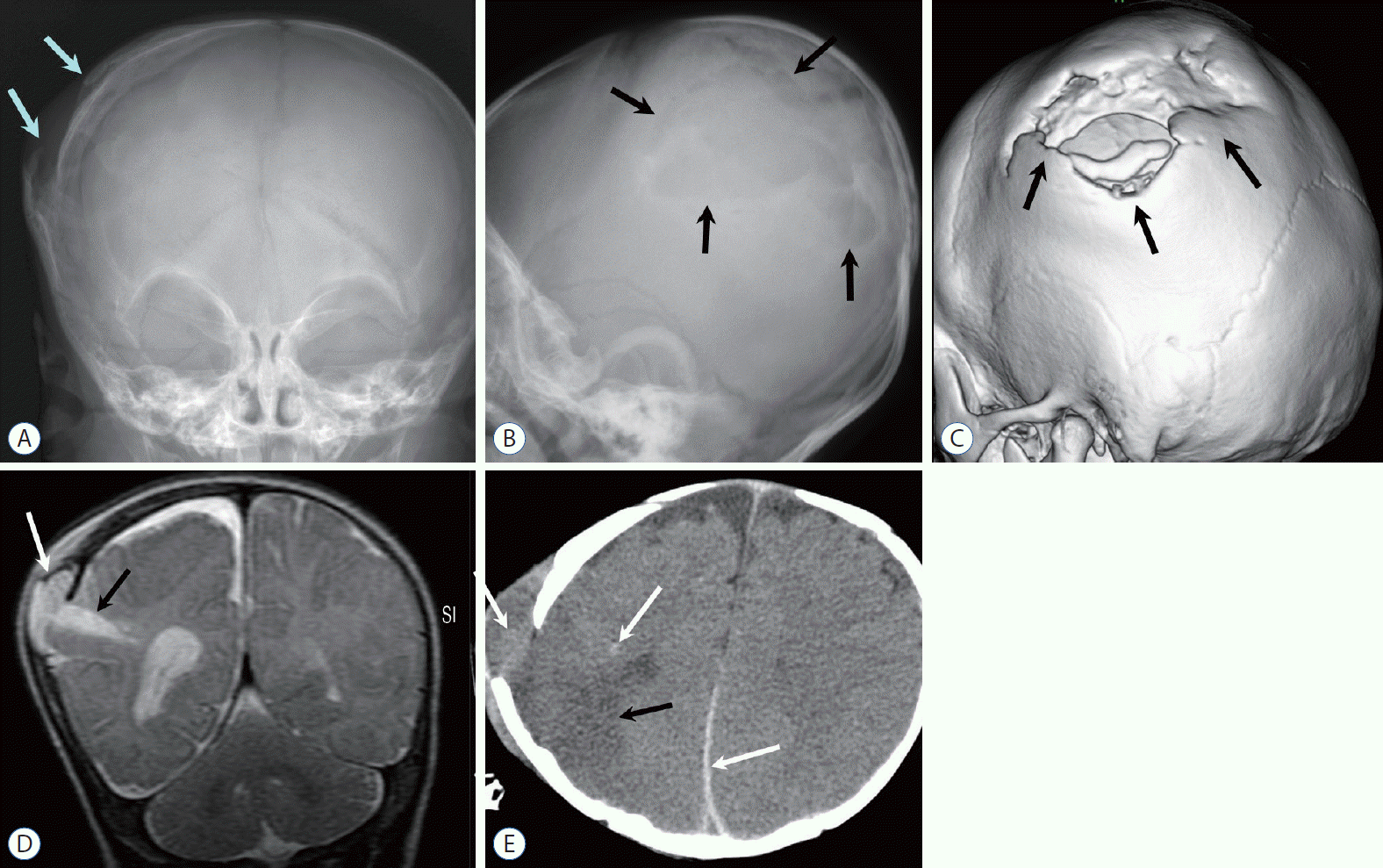
Fig. 7.
Six-month-old girl found in a comatose state at home. A : Skull anteroposterior radiography shows multiple skull fractures (arrows). B and C : Subsequent non-contrast-enhanced computed tomography images demonstrate extensive brain edema with high attenuation at the basal cistern (sign of pseudo-subarachnoid hemorrhage) and tentorial areas. Note multifocal small subdural hemorrhages (arrows) in the computed tomography (B) and sagittal T1-weighted image (D). E : T2-weighted image also shows massive cerebral swelling. Eye exam revealed retinal detachment and hemorrhage.
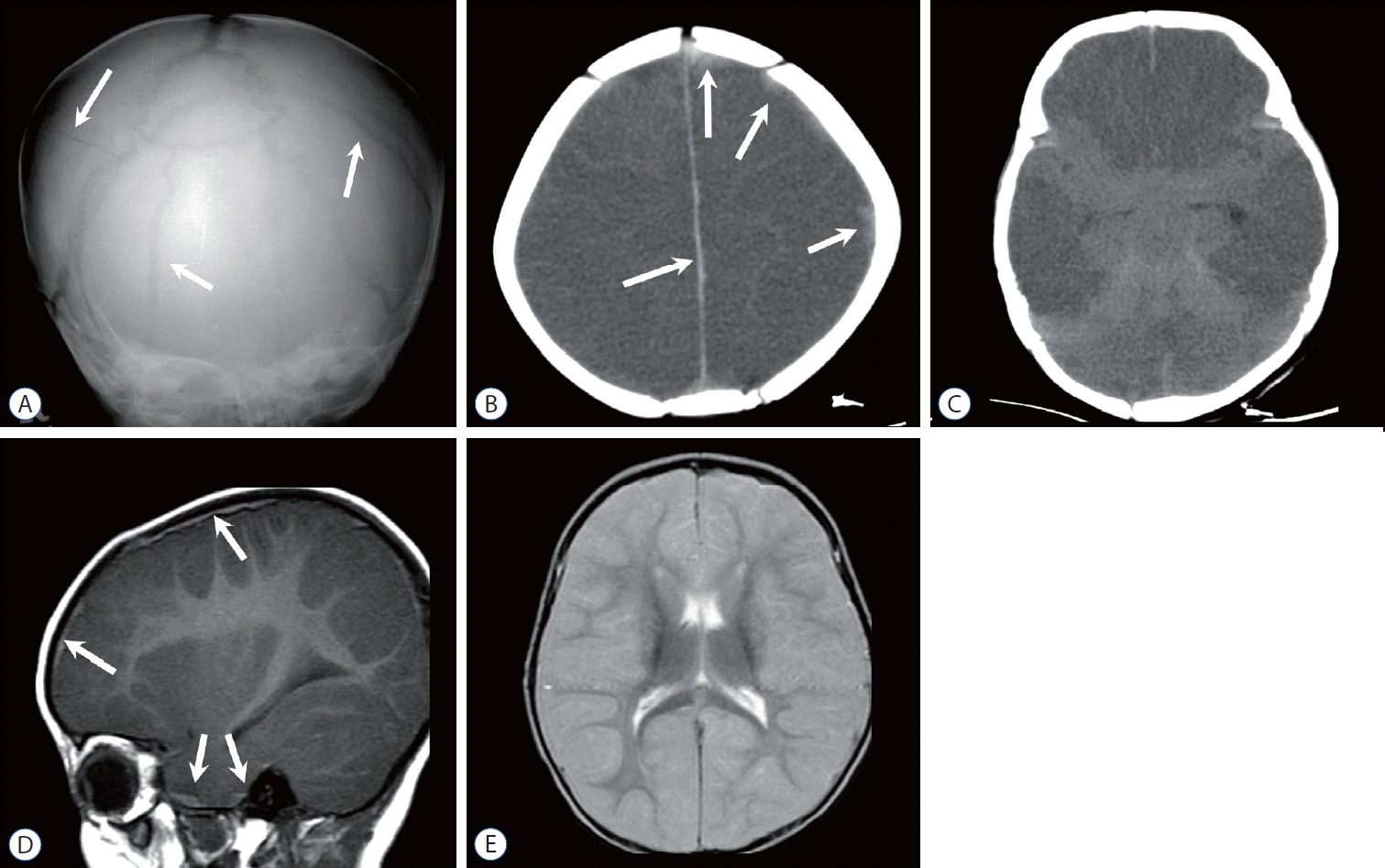
Fig. 8.
Neonate with presumed birth-related subdural hemorrhage (SDH). Sagittal T1-weighted image shows a small amount of SDH (arrow) in the posterior fossa.
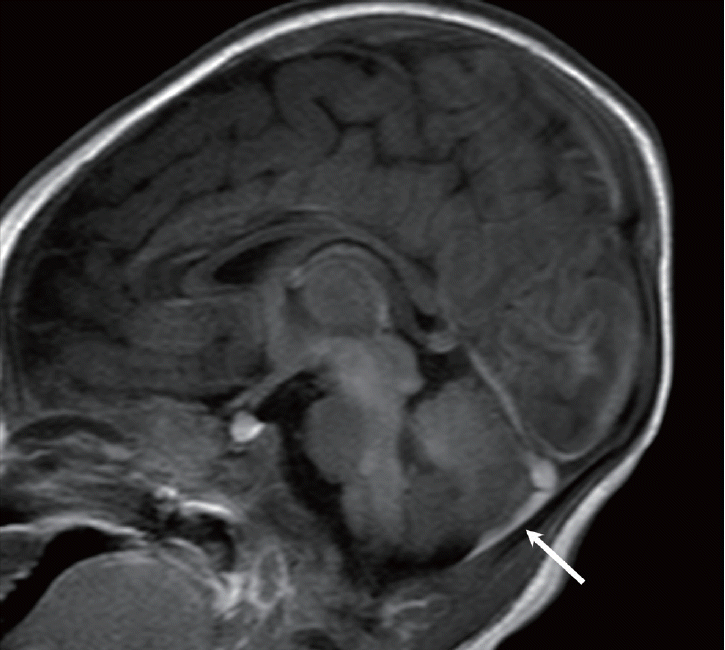
Fig. 9.
Infant with type 1 glutaric aciduria. A : Axial T2-weighted image (T2WI) shows bilateral subdural fluid collection (arrows). There are multifocal high signal intensity lesions at the globi pallidi, thalimi, and white matter. B : Typical hypoplastic opercula (arrows) became evident by the resolution of the subdural fluid on follow-up T2WI. There are additional parenchymal lesions exhibiting high intensity at the frontal white matter, thalami, and midbrain.
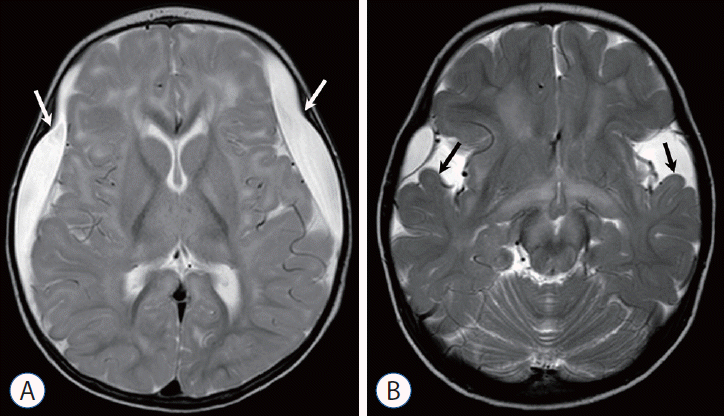
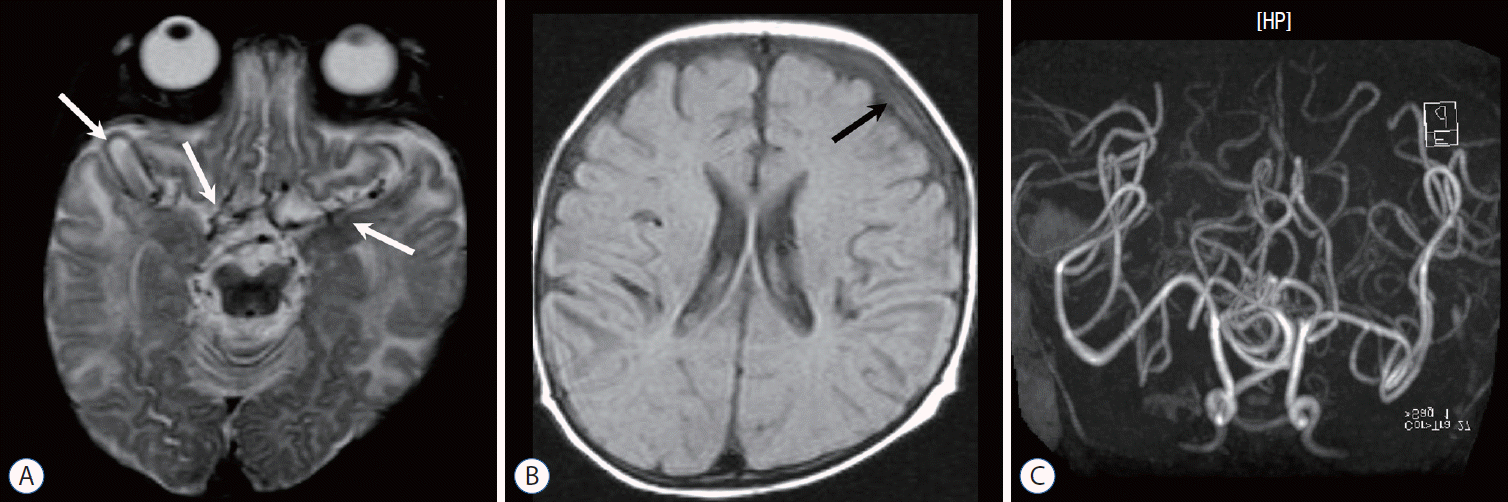
Fig. 10.
Infant with Menkes disease. A : T2-weighted image shows very tortuous arteries (arrows) and multifocal high intensity in the white matter. B : Fluid attenuated inversion recovery image shows a small amount of subdural hemorrhage (arrow) at the left convexity, which is associated with mild brain atrophy. C : Magnetic resonance angiography demonstrates the “kinky vessels,” a typical finding of the trichopoliodystrophy (Menkes disease).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download