1. Courbebaisse M, Cavalier E. Vitamin D in 2020: old pro-hormone with potential effects beyond mineral metabolism. Nutrients. 2020; 12:3378.
2. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001; 22:477–501. PMID:
11493580.
3. Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994; 60:619–630. PMID:
8092101.
4. Park HM, Kim JG, Choi WH, Lim SK, Kim GS. The vitamin D nutritional status of postmenopausal women in Korea. Korean J Bone Metab. 2003; 10:47–55.
5. Yamamoto M, Kawanobe Y, Takahashi H, Shimazawa E, Kimura S, Ogata E. Vitamin D deficiency and renal calcium transport in the rat. J Clin Invest. 1984; 74:507–513. PMID:
6086715.
6. Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001; 15:2579–2585. PMID:
11726533.
7. Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992; 13:719–764. PMID:
1333949.
8. Lemire JM. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 1995; 53:599–602. PMID:
7626516.
9. Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010; 20:182–190. PMID:
19807897.
10. Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008; 29:407–414. PMID:
18727936.
11. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281. PMID:
17634462.
12. Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Grantham N, Ebeling PR, Daly RM. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care. 2011; 34:1133–1138. PMID:
21430082.
13. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007; 92:2017–2029. PMID:
17389701.
14. Leu M, Giovannucci E. Vitamin D: epidemiology of cardiovascular risks and events. Best Pract Res Clin Endocrinol Metab. 2011; 25:633–646. PMID:
21872804.
15. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008; 117:503–511. PMID:
18180395.
16. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004; 80:1678S–88S. PMID:
15585788.
17. Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008; 20:532–537. PMID:
18698173.
18. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C.: National Academies Press;2011.
19. Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D
3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992; 327:1637–1642. PMID:
1331788.
20. Yamagata E, Yamada Y, Sugihara Y, Komatsu M, Kimura M, Okayama Y. Physical fitness and depression symptoms in community-dwelling elderly women. Nippon Koshu Eisei Zasshi. 2013; 60:231–240. PMID:
23909190.
21. Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB. Cardiovascular Health Study. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003; 123:387–398. PMID:
12576356.
22. Ho RC, Niti M, Kua EH, Ng TP. Body mass index, waist circumference, waist-hip ratio and depressive symptoms in Chinese elderly: a population-based study. Int J Geriatr Psychiatry. 2008; 23:401–408. PMID:
17879255.
23. Vogelzangs N, Kritchevsky SB, Beekman AT, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BW. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008; 65:1386–1393. PMID:
19047525.
24. Nah EH, Kim S, Cho HI. Vitamin D levels and prevalence of vitamin D deficiency associated with sex, age, region, and season in Koreans. Lab Med Online. 2015; 5:84–91.
25. da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem. 2007; 15:508–511. PMID:
17653438.
26. Kwon O, Kim H, Kim J, Hwang J, Lee J, Yoon MO. The development of the 2020 Dietary Reference Intakes for Korean population: lessons and challenges. J Nutr Health. 2021; 54:425–434.
27. Chung M, Balk EM, Ip S, Lee J, Terasawa T, Raman G, Trikalinos T, Lichtenstein AH, Lau J. Systematic review to support the development of nutrient reference intake values: challenges and solutions. Am J Clin Nutr. 2010; 92:273–276. PMID:
20504974.
28. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. PMID:
31462531.
29. Spill MK, English LK, Raghavan R, Callahan E, Güngör D, Kingshipp B, Spahn J, Stoody E, Obbagy J. Perspective: USDA nutrition evidence systematic review methodology: grading the strength of evidence in nutrition- and public health–related systematic reviews. Adv Nutr. 2021; nmab147. PMID:
34918032.
30. Tuck SP, Datta HK. Osteoporosis in the aging male: treatment options. Clin Interv Aging. 2007; 2:521–536. PMID:
18225452.
31. Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010; 25:1948–1957. PMID:
20499378.
32. Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009; 183:1–420.
33. Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010; 25:305–312. PMID:
19594303.
34. Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007; 158:1–235.
35. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009; 20:1807–1820. PMID:
19543765.
36. WHO Scientific Group on the Prevention and Management of Osteoporosis. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group. Geneva: World Health Organization;2003.
37. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C.: National Academies Press;2011.
38. Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010; 85:752–757. PMID:
20675513.
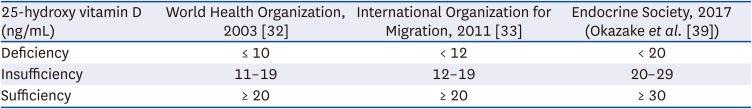
39. Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, Michigami T, Takeuchi Y, Matsumoto T, Sugimoto T. Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society [Opinion]. J Bone Miner Metab. 2017; 35:1–5. PMID:
27882481.
40. Jung SJ, Hwang BY, Jung JH, Kim HW, Jeong KH, Lee DW, Cho SH, Kim HW. Recent trends in serum vitamin D levels among Korean population: Korea National Health and Nutrition Examination Survey 2008∼2014. Korean J Clin Geriatr. 2018; 19:55–62.
41. Korea Meteorological Administration. Estimation of exposure time required for vitamin D production in human skin [Internet]. Daejeon: Korea Meteorological Administration;2016. cited 2021 June 10. Available from:
http://www.kma.go.kr.
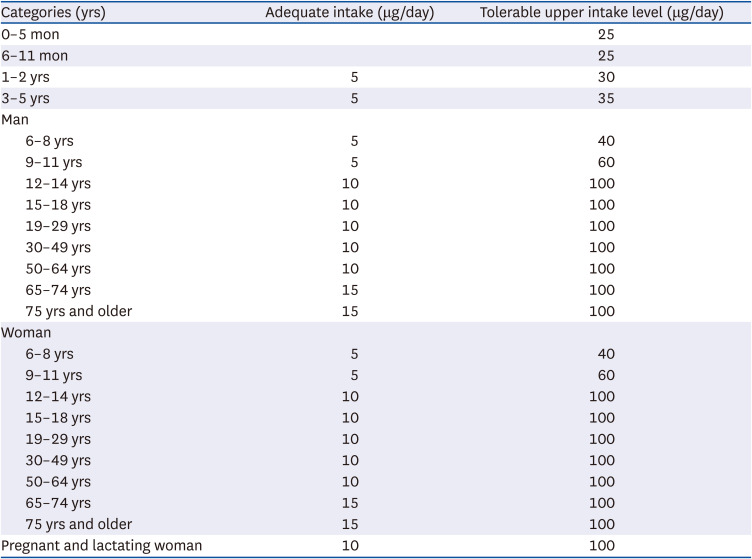
42. Food and Nutrition Board, Institute of Medicine (US). Dietary Reference Intakes for Calcium and Vitamin D. Washington D.C.: National Academy Press;2011.
43. Tamaki J, Iki M, Sato Y, Kajita E, Nishino H, Akiba T, Matsumoto T, Kagamimori S. JPOS Study Group. Total 25-hydroxyvitamin D levels predict fracture risk: results from the 15-year follow-up of the Japanese Population-based Osteoporosis (JPOS) cohort study. Osteoporos Int. 2017; 28:1903–1913. PMID:
28243705.
44. National Institute of Health and Nutrition of Japan. Dietary Reference Intakes for Japanese. Japan: National Institute of Health and Nutrition of Japan;2020.
45. Yu A, Kim J, Kwon O, Oh SY, Kim J, Yang YJ. The association between serum 25-hydroxyvitamin D concentration and consumption frequencies of vitamin D food sources in Korean adolescents. Clin Nutr Res. 2013; 2:107–114. PMID:
23908977.
46. Kim MJ. Nutritional status of vitamin D in Korean mothers and their babies and clinical study of vitamin D supplementation in breast-fed infants [doctor’s thesis]. Seoul: Seoul National University;2007.
47. The Korean Nutrition Society. Dietary Reference Intakes for Koreans. 1st revision. Seoul: The Korean Nutrition Society;2010.
48. Ministry of Health and Welfare (KR). The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2015. Sejong: Ministry of Health and Welfare;2015.
49. Steenbock H. The induction of growth promoting and calcifying properties in a ration by exposure to light. Science. 1924; 60:224–225. PMID:
17773313.
50. Food and Drug Administration. Agency information collection activities; submission for office of management and budget review; comment request; food labeling regulations. Fed Regist. 2009; 74:53743–53746.
51. Rural Development Administration (KR). National Institute of Agricultural Sciences (KR). The Korean food composition database 9.1. Jeonju: Rural Development Administration;2019. cited 2021 June 10. Available from:
http://www.koreanfood.rda.go.kr.
52. Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012; 367:40–49. PMID:
22762317.
53. Shimizu Y, Kim H, Yoshida H, Shimada H, Suzuki T. Serum 25-hydroxyvitamin D level and risk of falls in Japanese community-dwelling elderly women: a 1-year follow-up study. Osteoporos Int. 2015; 26:2185–2192. PMID:
25910748.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download