Abstract
Purpose
Intrahepatic cholangiocarcinoma (ICC) has various characteristics according to anatomical, histologic classifications, and its prognoses are different. This study aimed to compare oncologic outcomes according to tumor location (second bile duct confluence) and evaluate the effect of adjuvant chemotherapy.
Methods
Clinical data of 318 patients who underwent curative resection for ICC was reviewed. Central type ICC (C-ICC) and peripheral type ICC (P-ICC) were defined when the tumor invades the intrahepatic secondary biliary confluence and when located more peripherally, respectively.
Results
A larger tumor size, higher rate of elevated CA 19-9 level, vascular invasion, R1 resection, advanced T stage, and lymph node metastasis were found in C-ICC. C-ICC had poorer overall survival (median, 33 months vs. 58 months; P = 0.001), and the difference was more prominent in the early stage. C-ICC had a higher recurrence rate (68.7% vs. 55.1%, P = 0.014); otherwise, there was no difference in the recurrence patterns. There were no survival benefits of adjuvant chemotherapy in the entire cohort, but there were benefits in advanced stages (T3–4, N1 stage), especially in C-ICC.
Cholangiocarcinoma is a malignant tumor that arises from the bile duct, which is classified as intrahepatic cholangiocarcinoma (ICC), perihilar cholangiocarcinoma (PCC), and distal cholangiocarcinoma, depending on the location of the tumor. While ICC accounts for only 8%–10% of all bile duct cancers, the global incidence is gradually increasing [123]. ICC is the second most common primary liver cancer, following hepatocellular carcinoma (HCC) [4]. As ICC usually does not present with symptoms until advanced stages, the curative resection rate is only 20%–40% [5]. And even after surgery, ICC has an unfavorable prognosis with early, high recurrence rates [67].
Several methods have been used to classify the ICC. Based on the macroscopic growth pattern, it is classified into mass-forming, periductal infiltrating, and intraductal growing types [8]. According to the histological classification according to the level or size of the bile ducts where the tumor occurs, it is divided into small and large duct types [9]. As such, ICC contains a heterogeneous disease entity and these classifications are used to distinguish between different characteristics and to predict their prognosis. However, the classifications that result from pathologic reports are obtained after surgery; therefore, they cannot be used in deciding the surgical extent or preoperative treatment strategies.
ICC is defined as the location of the epicenter of the tumor is in the secondary branches or peripherally located interlobular bile ducts. However, in cases of ICC involving the hepatic hilum and PCC that involve the hepatic parenchyma, the range of the tumor can be partially overlapped. The principles of surgery differ between the ICC and PCC. In PCC, resection of the extrahepatic bile duct and regional lymph node dissection (LND) is essential, while in ICC, the surgical extent of the liver, extrahepatic bile duct, and lymph nodes remains controversial.
Even after curative resection, the recurrence rate is known high, so postoperative treatment has been attempted. However, the role of adjuvant chemotherapy in resected ICC has not been clarified, with limited studies due to its rare incidence, and optimal chemotherapeutic agents have yet to be determined [1011]. In addition, several studies are difficult to interpret because they include not only ICC but also extrahepatic bile duct and gallbladder cancer.
The aim of this study was to compare the clinicopathologic data and oncologic outcomes between ICC that involve the secondary bile duct confluence and ICC without any involvement. Additionally, we investigated the stage-specific prognosis and the benefits of adjuvant chemotherapy based on this classification.
This study was approved by the Institutional Review Board of the Seoul National University Hospital (No. SNUH 2102-112-119). The study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Patients who were confirmed to have ICC after curative liver resection at Seoul National University Hospital between 2003 and 2018 were included in this study. Diffuse bile duct cancer with intrahepatic invasion and Klatskin tumors were not included in this study. Patients who underwent palliative intent surgery, combined HCC-ICC, premalignant lesions (e.g., biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct), recurrent ICC, and 90-day postoperative mortality cases were excluded (Fig. 1).
The radiologic images and pathological reports of patients classified were reviewed if there were any tumor invasions to the left or right secondary bile duct confluence. Central type ICC (C-ICC) was defined when the tumor invades below the secondary biliary confluence, and peripheral type ICC (P-ICC) when the tumor was located more peripherally than the secondary confluence (Fig. 2).
The regions of recurrent tumors were classified as intrahepatic, locoregional, or distant recurrence. To focus on recurrences in the regional lymph nodes, recurrence at the surgical margin of the liver was classified as intrahepatic metastasis, and locoregional recurrence was defined as newly detected enlarged lymph nodes or soft tissues in the direction of lymphatic drainage based on tumor location. For right-sided ICCs, hilar, periduodenal, and peripancreatic lymph nodes are included in regional nodes, and for left-sided ICCs, hilar and gastrohepatic lymph nodes are included. Lymph nodes spread to the portocaval, aortocaval, and celiac regions were categorized as distant metastases.
This retrospective analysis was based on prospectively collected data. After curative resection of the primary tumor, the patients underwent regular follow-ups. Abdominal computed tomography or abdominal magnetic resonance imaging was performed every 3–6 months or when clinically indicated. The median follow-up duration was 36 months. Overall survival (OS) was calculated from the date of surgery. Patients who died 60 days or less after surgery were excluded because they may be related to complications of major liver resection.
Paired Student t-test was used to compare continuous variables, and the chi-square test and Fisher exact test were used for categorical variables. Kaplan-Meier curves with log-rank tests were used to compare survival rates. Variables were shown to be significantly associated (P < 0.05) in the univariate analysis using the multivariate Cox proportional hazards regression model to identify independent risk factors for survival. Estimates of the hazard ratio and its 95% confidence interval (CI) were reported. All statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA).
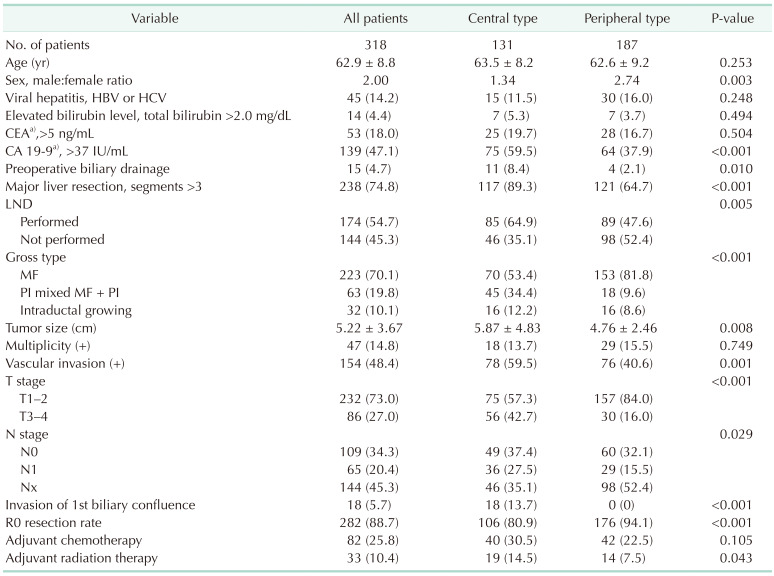
A total of 318 patients met the inclusion/exclusion criteria and were finally included in the study population (Fig. 1). The patients’ demographic and clinicopathological data are summarized in Table 1. There were 139 patients (47.1%) with an elevated CA 19-9 level (>37 IU/mL) and only 15 patients (4.7%) who received preoperative biliary drainage. As regional lymphadenectomy was not routinely performed in our institution, 174 patients (54.7%) underwent LND. The mean number of harvested lymph nodes was 5.1 ± 3.5 (range, 1–22) and lymph node metastatic rate was 37.4%. Only 5.7% had invasion of the hepatic hilum, 1st biliary confluence and 88.7% of patients had clear resection margins. Only 25.8 % of patients received adjuvant chemotherapy, but the use of adjuvant therapy has been on the rise in recent years compared to the past. Of patients, 57.3% received 5-FU based chemotherapy followed by gemcitabine-based chemotherapy as 40.2%.
When categorized by tumor location, 131 patients (41.2%) had C-ICC and 187 patients (58.8%) had P-ICC. A higher rate of elevated CA 19-9 levels (>37 IU/mL), major hepatectomies (89.3% vs. 64.7%), combined LND (64.9% vs. 47.6%), periductal infiltrative gross type, vascular invasion, advanced T stage (T3–4, 42.7% vs. 16.0%), lymph node positivity (42.4% vs. 32.6%), R1 resection (19.1% vs. 5.9%), and adjuvant treatment were examined in the central type. However, there were no differences in age, rate of HBV and HCV infection, elevated CEA level, proportion of intraductal growing type, and multiplicity of the tumor.
The median follow-up duration was 33 months and the median OS was 43.0 months (95% CI, 31.5–54.5 months). The survival at 5-years in the C-ICC group was worse than that in the P-ICC group (P = 0.001) (Fig. 3A). In the subgroup analysis of T stage, there was a survival difference between C-ICC and P-ICC in T1–2 (P = 0.039) (Fig. 3B), but not in T3–4 tumors (P = 0.824) (Fig. 3C). There was a survival difference between C-ICC and P-ICC in node-negative patients (P = 0.016) (Fig. 3D), but not in the node-positive group (P = 0.933) (Fig. 3E).
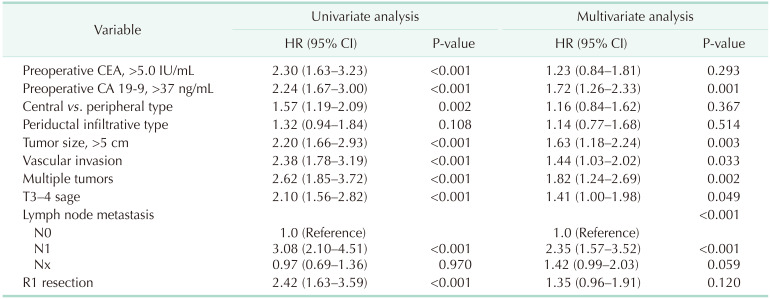
Tumor location (central vs. peripheral), preoperative tumor markers CEA and CA 19-9 level, and factors that are included in the American Joint Committee on Cancer (AJCC) 8th cancer staging system [12] such as tumor size of >5 cm, vascular invasion, multiplicity of tumors, perforation of visceral peritoneum (T3), extrahepatic involvement (T4), lymph node metastasis, and surgical margin status (R0 vs. R1) showed a significant difference in the univariate Cox regression analysis. In the multivariate Cox regression analysis of OS, CA 19-9 level (>37 ng/mL), tumor size (>5 cm), vascular invasion, multiplicity, extrahepatic involvement, and lymph node metastasis were identified as prognostic factors. (Table 2). Among the variables, lymph node positivity was the strongest factor (hazard ratio, 2.35; P < 0.001). However, preoperative CEA level (>5.0 IU/mL), tumor location (central vs. peripheral), periductal infiltrative type, and R1 status failed to show a significant difference.
In the subgroup analysis of central and peripheral types, different prognostic factors were observed (Supplementary Table 1). In C-ICC, tumor size (>5 cm), vascular invasion, and lymph node metastasis were risk factors for the central type. However, preoperative CA 19-9 level (>37 ng/mL), multiplicity of tumors, lymph node metastasis, and invasion of the surgical margin (R1) were risk factors for P-ICC.
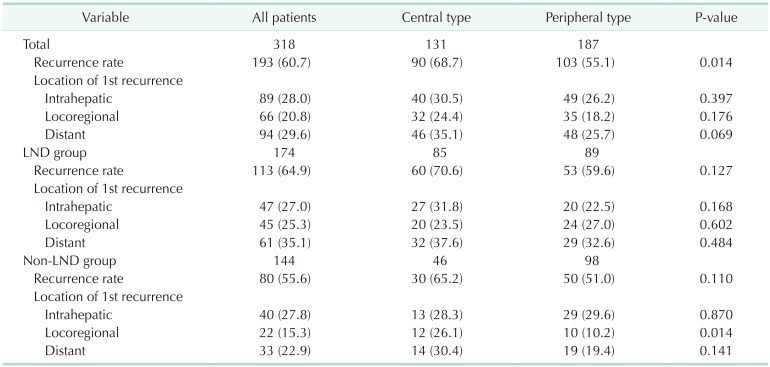
During the follow-up period, 193 patients (60.7%) developed cancer recurrence (Table 3). The median time to recurrence was 8 months (range, 1–85 months). Among the 193 patients, 89 (28.0%) developed recurrence in the remnant liver, 66 (20.8%) with locoregional recurrence, and 94 (29.4%) with distant metastasis. The recurrence rate of C-ICC was higher than that of P-ICC (68.7% vs. 55.1%, P = 0.014). However, there was no difference in the recurrence patterns.
Subgroup analysis according to the LND status was performed. The overall recurrence and locoregional recurrence rates in the LND group were 64.9% and 25.3%, respectively, which were higher than those in patients who did not undergo LND. In the LND group, there was no difference in the recurrence rate and recurrence patterns between the central and peripheral types. In contrast, there were more locoregional recurrences in C-ICC compared to P-ICC in the non-LND group (26.1% vs. 10.2%).
In all patients, 82 (25.8%) received adjuvant chemotherapy mainly in the advanced stages or R1 resected cases. There was no survival difference between the 2 groups (P = 0.097), and the median survival was longer in the no adjuvant chemotherapy group (44 months vs. 39 months) (Supplementary Fig. 1). However, there were survival benefits of adjuvant chemotherapy in T3–4 patients (P = 0.041) and N1 patients (P = 0.026) as well.
Further investigation was conducted on the effect of chemotherapy between T3–4, N1 C-ICC, and P-ICC patients (Fig. 4). While C-ICC had survival benefits from chemotherapy in T3–4 (P = 0.002) and N1 stage (P = 0.019), in P-ICC, there were none.
Different types of classifications have been used to understand the heterogeneous ICC features, and there are previous studies that have focused on tumor location. Several studies defined central type, or hilar type when the tumor involves the first bile duct confluence with poor prognostic features [1314]. Lee et al. [15] have reported results that tumors located near the first confluence (<4.5 cm) had a higher rate of node metastasis. After excluding tumors that have unclear boundaries to be classified as ICC or Klatskin tumor, only a small proportion (5.7%) had invasion of the hepatic hilum in the present study. Then, we evaluated the clinical significance of the second bile duct confluence, which can be evaluated using preoperative imaging and found meaningful results. The novel classification adopted in this study can be applied conveniently with preoperative images, and it seems to be advantageous for large tumors when the center of the tumor is difficult to identify.
ICC and PCC have different tumor characteristics, resulting in different treatment strategies, staging systems, and prognoses [1617]. It is often difficult to discriminate between ICC and PCC, when the tumor has both intra- and extrahepatic components, preoperatively. In addition, there are reports of misclassifications of ICC and extrahepatic bile duct cancers, there have been changes in the International Classification of Diseases coding system [1819]. Therefore, by carefully reviewing the radiologic images with pathologic reports, cases that should be classified as Klatskin tumor were excluded.
All of the T category factors in the AJCC 8th edition staging system [12] were identified as risk factors in this study, and lymph node metastasis was the strongest prognostic risk factor, in agreement with previous studies. Periductal infiltrating type did not have clinical impact on worse survival. As routine LND were not performed in our institution, it was not possible to classify the accurate cancer stage and lymph node profile. As this study focused on the tumor location of ICC, analysis related to lymph node status is being prepared in subsequent papers. In a comparison of the risk factors between C-ICC and P-ICC, preoperative CA 19-9 (>37 ng/mL) and lymph node metastasis were common risk factors, and other factors showed different results, indicating that they have different tumor characteristics.
The rate of lymph node metastasis in resected ICC is known up to be 30%–50% [2021], but the oncologic benefits of LND still seem to be controversial [2223]. Several studies have suggested lymph node metastasis prediction models with preoperative clinical factors [2425], and it is cautious to determine in which case LND can be omitted. The high locoregional recurrence rate in the C-ICC, and non-LND groups suggests that LND is essential, especially in the central type.
ICC recurrence after curative intent surgery is known to be as high as 55%–74.3% and intrahepatic recurrence is known to be the most common [2627]. To evaluate the relationship between LND and locoregional recurrence, the definition was limited to the regional lymph node territory based on tumor location, and recurrence at the liver resection margin was regarded as intrahepatic recurrence. In the subgroup analysis of the non-LND group, a higher incidence of locoregional recurrence was detected in C-ICC patients than in P-ICC, and the significance of tumor location (central vs. peripheral) in multivariate regression analysis implies that LND should be routinely performed in C-ICC.
Studies of adjuvant treatment focused on ICC are rare and usually include patients with gallbladder cancer and other biliary tract cancers. A previous meta-analysis of 5,060 patients reported an improvement in OS with adjuvant chemotherapy compared with surgery alone in advanced diseases [28], which was consistent with the results of this study. However, the 2 randomized clinical trials of adjuvant chemotherapy in biliary tract cancer failed to achieve significant results of survival benefit [2930]. As ICC has different tumor characteristics from other biliary tract cancers, large-volume prospective studies and randomized clinical trials focused on the adjuvant treatment of ICC are needed.
There are some limitations in this study. First, the current study is a retrospective single-center study that may have included some potential confounding factors and bias. To diminish these factors, medical records were reviewed and integrated by specialists, and cases with ambiguous boundaries or poor data were excluded from the study. Second, although the classification method used in the current study stratified clearly, there were some vague aspects of the existence of various bile duct anatomy variations. At last, less than 30% of patients received adjuvant chemotherapy after surgery, because routine administration was not performed but the number of patients receiving postoperative treatment is gradually increasing followed by recent studies.
In conclusion, the central type has more aggressive tumor characteristics with worse prognosis than the peripheral type, and the tumor location (involvement of the second biliary confluence) has prognostic significance in resected ICC. Given that the lymph node metastasis rate is higher in C-ICC and the locoregional recurrence rate is higher in non-LND and C-ICC patients, LND should be performed in the central type. Adjuvant chemotherapy is effective in advanced T stage and node-positive patients, especially in the central type.
References
1. De Oliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007; 245:755–762. PMID: 17457168.
2. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014; 383:2168–2179. PMID: 24581682.
3. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016; 21:594–599. PMID: 27000463.
4. Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009; 208:134–147. PMID: 19228515.
5. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014; 60:1268–1289. PMID: 24681130.
6. Hu LS, Zhang XF, Weiss M, Popescu I, Marques HP, Aldrighetti L, et al. Recurrence patterns and timing courses following curative-intent resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2019; 26:2549–2557. PMID: 31020501.
7. Kojima T, Umeda Y, Fuji T, Niguma T, Sato D, Endo Y, et al. Efficacy of surgical management for recurrent intrahepatic cholangiocarcinoma: a multi-institutional study by the Okayama Study Group of HBP surgery. PLoS One. 2020; 15:e0238392. PMID: 32881910.
8. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003; 10:288–291. PMID: 14598147.
9. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010; 2:419–427. PMID: 21191517.
10. Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: a systematic review and meta-analysis. PLoS One. 2020; 15:e0229292. PMID: 32084210.
11. Zhu AX, Knox JJ. Adjuvant therapy for intrahepatic cholangiocarcinoma: the debate continues. Oncologist. 2012; 17:1504–1507. PMID: 23220842.
12. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer;2017.
13. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Sueda T. Intrahepatic cholangiocarcinoma: clinicopathological differences between peripheral type and hilar type. J Gastrointest Surg. 2012; 16:540–548. PMID: 22012305.
14. Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007; 31:1059–1067. PMID: 17592273.
15. Lee W, Jeong CY, Jang JY, Roh YH, Kim KW, Kang SH, et al. Clinical implication of tumor site in terms of node metastasis for intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2020; 46:832–838. PMID: 31806519.
16. Lu J, Li B, Li FY, Ye H, Xiong XZ, Cheng NS. Long-term outcome and prognostic factors of intrahepatic cholangiocarcinoma involving the hepatic hilus versus hilar cholangiocarcinoma after curative-intent resection: should they be recognized as perihilar cholangiocarcinoma or differentiated? Eur J Surg Oncol. 2019; 45:2173–2179. PMID: 31208772.
17. Zhang XF, Bagante F, Chen Q, Beal EW, Lv Y, Weiss M, et al. Perioperative and long-term outcome of intrahepatic cholangiocarcinoma involving the hepatic hilus after curative-intent resection: comparison with peripheral intrahepatic cholangiocarcinoma and hilar cholangiocarcinoma. Surgery. 2018; 163:1114–1120. PMID: 29398035.
18. Kang MJ, Lim J, Han SS, Park HM, Kim SW, Won YJ, et al. Impact of changes in the topographic classification of Klatskin tumor on incidence of intra- and extrahepatic bile duct cancer: a population-based national cancer registry study. J Hepatobiliary Pancreat Sci. 2021; 28:740–750. PMID: 33615747.
19. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019; 39 Suppl 1:19–31. PMID: 30851228.
20. Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann Surg. 2021; 274:e1187–e1195. PMID: 31972643.
21. Bagante F, Gani F, Spolverato G, Xu L, Alexandrescu S, Marques HP, et al. Intrahepatic cholangiocarcinoma: prognosis of patients who did not undergo lymphadenectomy. J Am Coll Surg. 2015; 221:1031–1040. PMID: 26474514.
22. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016; 18:79–87. PMID: 26776855.
23. Li DY, Zhang HB, Yang N, Quan Y, Yang GS. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol. 2013; 19:9084–9091. PMID: 24379635.
24. Tsilimigras DI, Sahara K, Paredes AZ, Moro A, Mehta R, Moris D, et al. Predicting lymph node metastasis in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2021; 25:1156–1163. PMID: 32757124.
25. Hu J, Chen FY, Zhou KQ, Zhou C, Cao Y, Sun HC, et al. Intrahepatic cholangiocarcinoma patients without indications of lymph node metastasis not benefit from lymph node dissection. Oncotarget. 2017; 8:113817–113827. PMID: 29371948.
26. Zhang XF, Chakedis J, Bagante F, Beal EW, Lv Y, Weiss M, et al. Implications of intrahepatic cholangiocarcinoma etiology on recurrence and prognosis after curative-intent resection: a multi-institutional study. World J Surg. 2018; 42:849–857. PMID: 28879598.
27. Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013; 153:811–818. PMID: 23499016.
28. Ma KW, Cheung TT, Leung B, She BW, Chok KS, Chan AC, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: a meta-analysis. Medicine (Baltimore). 2019; 98:e14013. PMID: 30702559.
29. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019; 20:663–673. PMID: 30922733.
30. Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019; 37:658–667. PMID: 30707660.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 and Supplementary Fig. 1 can be found via https://doi.org/10.4174/astr.2022.102.5.248.
Supplemental Table 1
Independent risk factors for overall survival in central and peripheral type intrahepatic cholangiocarcinoma
Supplementary Fig. 1
Effect of adjuvant chemotherapy on survival after curative resection of intrahepatic cholangiocarcinoma in entire patients (A), T3-4 (B), and N1 (C) patients.
Fig. 1
Flow chart of patient selection. ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma.
Fig. 2
Schematic anatomy of central and peripheral type intrahepatic cholangiocarcinoma. The dotted line is an extension of the left and right secondary bile duct confluence. Central type refers to tumors where there is invasion to the secondary bile duct confluence or is located below the secondary bile duct confluence and peripheral type does not have any invasion.
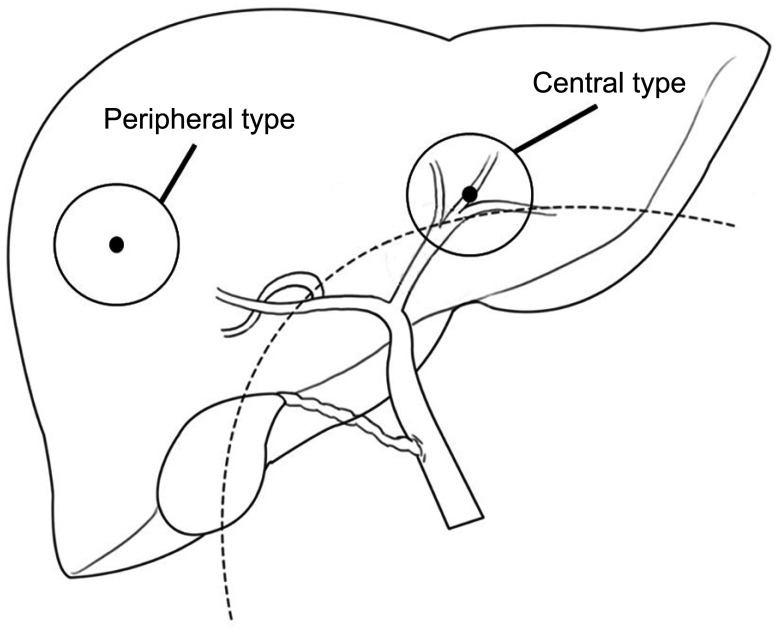
Fig. 3
Overall survival for central and peripheral type intrahepatic cholangiocarcinoma in entire patients and specific T, N stages. Central type had poorer survival compared to peripheral type in (A) entire study population (P = 0.001), (B) T1–2 (P = 0.039), and (D) N0 (P = 0.016) patients. On the contrary, there was no survival difference in (C) T3–4 (P = 0.824) and (E) N1 (P = 0.933) patients.
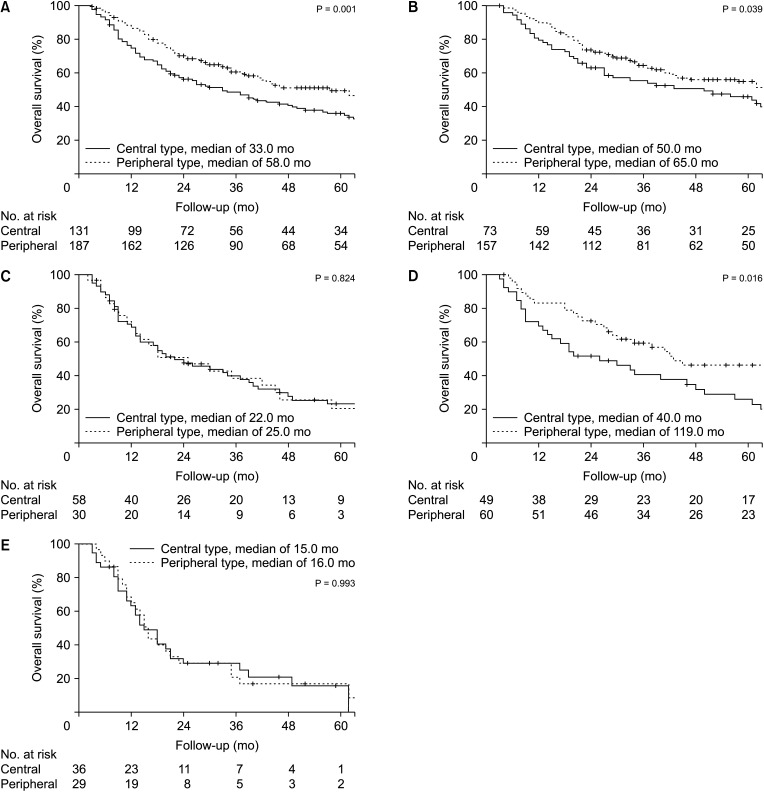
Fig. 4
Effect of adjuvant chemotherapy on survival after curative resection of intrahepatic cholangiocarcinoma in T3–4 and N1 patients. Central type T3–4 (A) had survival benefits of adjuvant chemotherapy (P = 0.002), while peripheral type T3–4 (B) did not (P = 0.425). Central type N1 (C) had survival benefits of adjuvant chemotherapy (P = 0.019), while peripheral N1 type did not (P = 0.463).
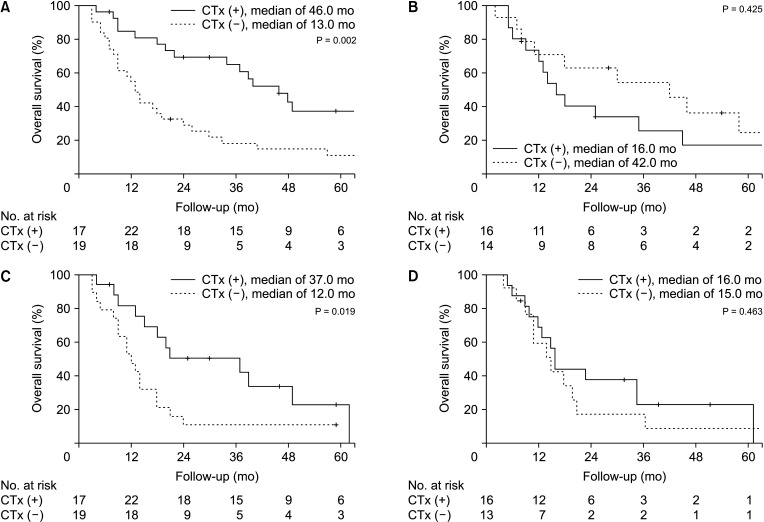
Table 1
Clinicopathological data of central type and peripheral type intrahepatic cholangiocarcinoma




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download