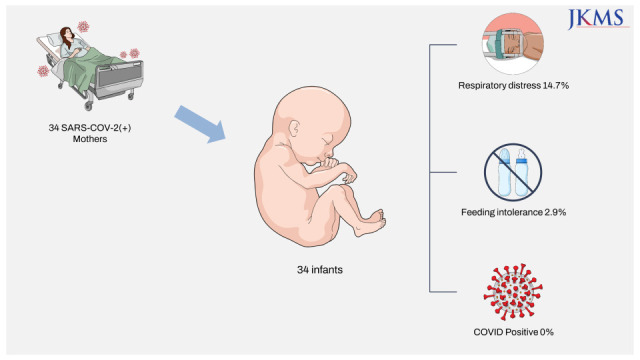
Abstract
With the spread of coronavirus disease 2019 (COVID-19) in Korea, the number of pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is rapidly increasing. A shortage of negative-pressure isolation rooms for newborns makes hospital assignment more difficult for late-pregnant women with COVID-19. Among 34 infants born to SARS-CoV-2-positive mothers, 5 (14.7%) presented with respiratory distress and 1 (2.9%) presented with feeding intolerance that required specialized care. Aerosol-generating procedures were performed in one infant. Overall outcomes of 34 infants were favorable, and no infant tested positive for SARS-CoV-2. Most infants born to SARS-CoV-2-positive mothers did not need to be quarantined in a negative-pressure isolation room, and 17 (50%) mother–infant dyads were eligible for rooming-in. If negative-pressure isolation rooms are selectively used for newborns requiring aerosol-generating procedures or newborns in respiratory distress, resource availability for lower-risk cases may improve.
Graphical Abstract
By August 2021, the cumulative number of pregnant women with coronavirus disease 2019 (COVID-19) in South Korea was 731 (< 0.3% of all cases).1 Nevertheless, few hospitals admitted late-pregnant women with COVID-19. Since the autumn of 2021, the number of COVID-19-affected pregnant women has increased, and the delay in bed assignment emerged as a social problem.
Newborns of mothers with COVID-19 are assumed to have severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection until test results are available. Isolation care requirements of newborns make hospital allocation for late-pregnant women with COVID-19 difficult. Although rooming-in is promoted in many guidelines,2345 a neonatal intensive care unit (NICU) is the only suitable environment for appropriate care of an isolated neonate in most hospitals in Korea. Therefore, Korean guidelines recommend that newborns should be immediately isolated in a negative-pressure room or a private room in the NICU if no negative-pressure rooms are available.6 Considering exposure risks in NICUs, clinicians are reluctant to place newborns with maternal COVID-19 in non-negative-pressure rooms. Given the on-going COVID-19 pandemic and limited resources, the risk of intrauterine and perinatal SARS-CoV-2 transmission and need for negative-pressure isolation rooms should be examined. This study aimed to describe clinical characteristics and hospital courses of infants born to SARS-CoV-2-positive mothers and suggest ways to resolve the shortage of isolation rooms in this context.
The management of labor and delivery of pregnant women with COVID-19 at the National Medical Center was based on the latest Korean Society of Pediatric Infectious Diseases guidelines.7 All mothers with COVID-19 delivered via cesarean section in isolation in a negative-pressure operating room. The newborn resuscitation area was 2 m away from the mother. Mothers wore masks during the delivery. After routine resuscitation (drying, stimulation, and suctioning) and additional resuscitative measures, as required, newborns were moved in a closed transport incubator to private negative-pressure rooms in the nursery and were separated from unaffected newborns. Newborn evaluation and care, SARS-CoV-2 testing, and criteria for discontinuing isolation were performed according to the latest guidelines.7 After 2 consecutive negative SARS-CoV-2 polymerase chain reaction (PCR) tests on nasopharyngeal and oropharyngeal samples at 24 hours and 48 hours after birth, newborns with maternal COVID-19 were released from quarantine and cared for with other newborns in the nursery until discharge. Infants were fed formula milk during maternal isolation.
Between December 2020 and December 2021, 34 women with gestational age ≥ 35 weeks delivered 34 newborns during their isolation period. According to the U.S. National Institutes of Health COVID-19 Treatment Guidelines criteria,8 7 (20.6%) cases had severe COVID-19 before delivery and 5 (14.7%) cases progressed to severe illness after delivery. A total of 15 (44.1%) postpartum women had severe or critical COVID-19 or body temperature ≥ 38.0°C. Mother and newborn characteristics are presented in Table 1.
The median gestational age at birth was 38.3 weeks. Six (17.6%) infants were born preterm. No infants required advanced resuscitation such as chest compressions, positive-pressure ventilation, or medication at birth. During the hospital stay, 6 (17.6%) infants required specialized care. One infant with feeding intolerance required intravenous fluid therapy. Five infants had respiratory distress and required respiratory support: 4 with supplemental oxygen via a nasal cannula and 1 with nasal continuous positive airway pressure. The final diagnoses were transient tachypnea of the newborn (n = 3), delayed transition (n = 1), and meconium aspiration syndrome (n = 1). The infants with respiratory distress improved shortly and were weaned to room air within 48 hours of age. Overall, 17 (50.0%) mother–infant dyads (mothers with mild or moderate illness and without fever and infants not requiring specialized care) remained well during the entire hospitalization.
The SARS-CoV-2 PCR test results of all 34 newborns were negative at 24 hours and 48 hours after birth. After release from a median of 3 days (interquartile range, 2–3 days) of quarantine, the newborns were cared for in the nursery with non-COVID-19-exposed newborns. The 23 (67.6%) newborns stayed in the nursery until their mother was discharged. On follow-up phone calls made by nurses within 7 days after discharge, all infants remained in good condition and there were no additional confirmed cases of COVID-19 among them.
Previous reports on newborns of mothers with COVID-19 in Korea reported about 4 infants born at full term via cesarean section and immediately separated from their mothers.91011 No infant tested positive for SARS-CoV-2. Our findings are consistent with those of previous case studies managed using a similar policy. Worldwide, the mother-to-infant transmission rate of SARS-CoV-2 is reported to be in the range of 7–13%1213 based on studies published before the implementation of the current guidelines, potentially overestimating the true incidence. In contrast, U.S. studies with larger sample sizes reported virtually no mother-to-infant transmission of SARS-CoV-2, despite high rates of rooming-in and direct breastfeeding.14151617
An infant born to a mother with COVID-19 and requiring neonatal intensive care and respiratory support should be admitted to a private room with the potential for negative room pressure or to one with a door.218 For an infant with mild respiratory distress, which does not require positive pressure ventilation, a private room may be an adequate preventive strategy.18 Under a more stringent policy, stable newborns with respiratory support of up to 2 L/min of oxygen via a nasal cannula can be placed in neutral pressure rooms with precautions.19 According to these criteria, only 1 (2.9%) infant in our study required a negative-pressure isolation room. Approximately 12–15% of infants of mothers with COVID-19 were admitted to the NICU in previous studies including early preterm infants.151620 The proportion of infants requiring aerosol generating procedures (AGPs) is estimated to be in the range of < 12–15% and even lower for late-preterm and full-term infants. Because intrauterine infection of SARS-CoV-2 is rare, and the risk of postnatal transmission is low while rooming-in with appropriate precautions,17 the risk of airborne SARS-CoV-2 transmission in newborns, independently of being separated from the mother immediately after birth, is likely very low. If negative-pressure isolation rooms are selectively used for newborns requiring AGPs, resource availability for lower-risk cases may improve.
Half of the mother–infant dyads in our study remained well during their hospital stay. Rooming-in, if practiced, might have helped relieve the burden of hospital overcrowding because it requires a single patient room for the mother and infant. Mother-to-infant transmission of SARS-CoV-2 during rooming-in is rare, provided that adequate droplet and contact precautions are taken. In studies evaluating rooming-in for infants born to SARS-CoV-2-positive mothers, most mother–infant dyads safely roomed-in.141517
,
212223 Separating mothers and newborns may not be warranted to prevent SARS-CoV-2 transmission. Based on accumulating evidence and given the benefits of breastfeeding and mother–infant contact, many guidelines recommend rooming-in unless precluded by the mother or newborn condition.2345 In general, mothers are eligible for rooming-in under the following conditions: no need for respiratory support or supplemental oxygen, body temperature < 38°C, stable vital signs, and ability to take care of the baby. Newborns are eligible for rooming-in when they are well-appearing, with a gestational age ≥ 35 weeks and birth weight ≥ 2,000 g, physical examination findings within normal limits, vital signs within the reference ranges, and skilled in feeding.1523 Temporary separation of the newborn from the mother during hospital stay may be inevitable for either maternal or neonatal reasons. When rooming-in is interrupted, the newborn may be isolated in a private room unless AGPs are required.
Our study had some limitations. First, this was a single-center study. Our results should be interpreted with caution because they may have been influenced by the strict policy implemented at our institution. Second, this study did not include cases requiring neonatal intensive care for maternal or fetal indications, thus limiting the generalizability of the results to pregnant women with initially severe COVID-19 illness, with a gestation age of 21–34 weeks and early preterm infants, or with a high-risk fetus. Despite these limitations, this study presented data on the largest number of SARS-CoV-2-positive mother–newborn dyads in Korea to date. Furthermore, our study suggests that most infants with gestational age ≥ 35 weeks can be safely isolated in a private room and are clinically well enough to room-in with their mother. A negative-pressure room can be spared for infants requiring AGPs. The change in isolation policy may help resolve the shortage of newborn isolation rooms and ensure safe delivery by mothers during the COVID-19 pandemic. Further multicenter studies with larger patient cohorts are required to elucidate the characteristics and outcomes of SARS-CoV-2-positive women and their newborns in South Korea.
ACKNOWLEDGMENTS
We would like to thank all of the staffs of the newborn nursery and delivery units of National Medical Center.
References
1. Korean Disease Control and Prevention Agency. Coronavirus disease-19 press release. Updated 2021. Accessed January 8, 2022.
https://kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=717155&act=view
.
2. American Academy of Pediatrics. FAQs: management of infants born to mothers with suspected or confirmed COVID-19. Updated 2021. Accessed January 8, 2022.
https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/
.
3. Canadian Paediatric Society. Breastfeeding and COVID-19. Updated 2021. Accessed January 8, 2022.
https://cps.ca/en/documents/position/breastfeeding-when-mothers-have-suspected-or-proven-covid-19
.
4. World Health Organization. Breastfeeding and COVID-19. Updated 2020. Accessed January 8, 2022.
https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Breastfeeding-2020.1
.
5. Yeo KT, Oei JL, De Luca D, Schmölzer GM, Guaran R, Palasanthiran P, et al. Review of guidelines and recommendations from 17 countries highlights the challenges that clinicians face caring for neonates born to mothers with COVID-19. Acta Paediatr. 2020; 109(11):2192–2207. PMID: 32716579.
6. Kim KH, Cho EY, Kim DH, Kim HW, Park JY, Eun BW, et al. Guidelines for coronavirus disease 2019 response in children and adolescents. Pediatr Infect Vaccine. 2020; 27(1):24–34.
7. Korean Society of Pediatric Infectious Diseases. Guidelines for coronavirus disease 2019 response in children and adolescents. Updated 2020. Accessed January 8, 2022.
http://www.kspid.or.kr/board/list.html?code=notice&num=587
.
8. National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated 2022. Accessed February 6, 2022.
https://www.covid19treatmentguidelines.nih.gov/
.
9. Lee EK, Kim WD, Lee DW, Lee SA. Management of the first newborn delivered by a mother with COVID-19 in South Korea. Clin Exp Pediatr. 2020; 63(9):373–375. PMID: 32683810.
10. Bae JG, Ha JK, Kwon M, Park HY, Seong WJ, Hong SY. A case of delivery of a pregnant woman with COVID-19 infection in Daegu, Korea. Obstet Gynecol Sci. 2020; 63(6):745–749. PMID: 33012160.
11. Jin JH, Kim Y, Yoo J, Kim EH, Yoon SW. Two cases of SARS-CoV-2-positive mothers and their newborns in Korea. Infect Chemother. 2021; 53:e41.
12. Trippella G, Ciarcià M, Ferrari M, Buzzatti C, Maccora I, Azzari C, et al. COVID-19 in pregnant women and neonates: a systematic review of the literature with quality assessment of the studies. Pathogens. 2020; 9(6):485.
13. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021; 175(8):817–826. PMID: 33885740.
14. Angelidou A, Sullivan K, Melvin PR, Shui JE, Goldfarb IT, Bartolome R, et al. Association of maternal perinatal SARS-CoV-2 infection with neonatal outcomes during the COVID-19 pandemic in Massachusetts. JAMA Netw Open. 2021; 4(4):e217523. PMID: 33890989.
15. Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health. 2020; 4(10):721–727. PMID: 32711687.
16. Verma S, Bradshaw C, Auyeung NS, Lumba R, Farkas JS, Sweeney NB, et al. Outcomes of maternal-newborn dyads after maternal SARS-CoV-2. Pediatrics. 2020; 146(4):e2020005637. PMID: 32737153.
17. Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2021; 175(2):157–167. PMID: 33044493.
18. Canadian Paediatric Society. NICU care for infants born to mothers with suspected or confirmed COVID-19. Updated 2021. Accessed January 8, 2022.
https://cps.ca/documents/position/nicu-care-for-infants-born-to-mothers-with-suspected-or-proven-covid-19
.
19. Verma S, Lumba R, Lighter JL, Bailey SM, Wachtel EV, Kunjumon B, et al. Neonatal intensive care unit preparedness for the novel coronavirus disease-2019 pandemic: a New York City hospital perspective. Curr Probl Pediatr Adolesc Health Care. 2020; 50(4):100795. PMID: 32410913.
20. Gurol-Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021; 225(5):522.e1–522.11. PMID: 34023315.
21. Capozza M, Salvatore S, Baldassarre ME, Inting S, Panza R, Fanelli M, et al. Perinatal transmission and outcome of neonates born to SARS-CoV-2-positive mothers: The experience of 2 highly endemic Italian regions. Neonatology. 2021; 118(6):665–671. PMID: 34628414.
22. Flaherman VJ, Afshar Y, Boscardin WJ, Keller RL, H Mardy A, Prahl MK, et al. Infant outcomes following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): First report from the pregnancy coronavirus outcomes registry (PRIORITY) study. Clin Infect Dis. 2021; 73(9):e2810–e2813. PMID: 32947612.
23. Ronchi A, Pietrasanta C, Zavattoni M, Saruggia M, Schena F, Sinelli MT, et al. Evaluation of rooming-in practice for neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection in Italy. JAMA Pediatr. 2021; 175(3):260–266. PMID: 33284345.
Table 1
Demographic and clinical characteristics of late-preterm and full-term infants born to SARS-CoV-2-positive mothers
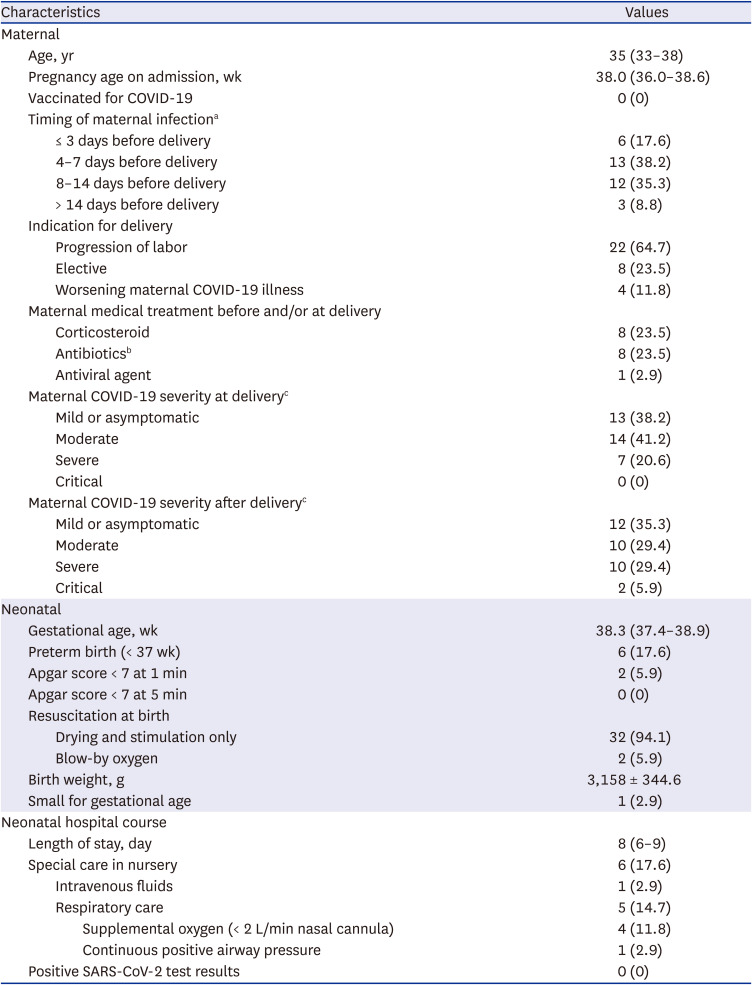
Values are presented as median (interquartile range), number (%) or mean ± SD.
COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
aSARS-CoV-2 infection date was defined as the date of symptom onset or the first positive polymerase chain reaction test result, whichever came first.
bAntibiotics used for surgical prophylaxis were excluded.
cSARS-CoV-2 Illness Severity Criteria were adapted from the U.S. National Institutes of Health COVID-19 Treatment Guidelines. Mild Illness: Individuals with signs and symptoms of COVID-19 but without dyspnea or abnormal chest imaging. Moderate Illness: Individuals with evidence of lower respiratory disease during clinical assessment or imaging and an oxygen saturation (SpO2) of ≥ 94% in room air at sea level. Severe Illness: Individuals with an SpO2 of < 94% in room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, respiratory rate > 30 breaths/min, or lung infiltrates > 50%. Critical Illness: Individuals with respiratory failure, septic shock, and/or multiple organ dysfunction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download