Abstract
Objective
The beneficial effect of hypothermia after hemicraniectomy in malignant middle cerebral artery (MCA) infarction has been controversial. We aim to investigate the safety and clinical efficacy of hypothermia after hemicraniectomy in malignant MCA infarction.
Methods
From October 2012 to February 2016, 20 patients underwent hypothermia (Blanketrol III, Cincinnati Sub-Zero, Cincinnati, OH, USA) at 34°C after hemicraniectomy in malignant MCA infarction (hypothermia group). The indication of hypothermia included acute cerebral infarction >2/3 of MCA territory and a Glasgow coma scale (GCS) score <11 with a midline shift >10 mm or transtentorial herniation sign (a fixed and dilated pupil). We retrospectively collected 27 patients, as the control group, who had undergone hemicraniectomy alone and simultaneously met the inclusion criteria of hypothermia between January 2010 and September 2012, before hypothermia was implemented as a treatment strategy in Dong-A University Hospital. We compared the mortality rate between the two groups and investigated hypothermia-related complications, such as postoperative bleeding, pneumonia, sepsis and arrhythmia.
Results
The age, preoperative infarct volume, GCS score, National institutes of Health Stroke Scale score, and degree of midline shift were not significantly different between the two groups. Of the 20 patients in the hypothermia group, 11 patients were induced with hypothermia immediately after hemicraniectomy and hypothermia was initiated in 9 patients after the decision of hypothermia during postoperative care. The duration of hypothermia was 4±2 days (range, 1 to 7 days). The side effects of hypothermia included two patients with arrhythmia, one with sepsis, one with pneumonia, and one with hypotension. Three cases of hypothermia were discontinued due to these side effects (one sepsis, one hypotension, and one bradycardia). The mortality rate of the hypothermia group was 15.0% and that of the control group was 40.7% (p=0.056). On the basis of the logistic regression analysis, hypothermia was considered to contribute to the decrease in mortality rate (odds ratio, 6.21; 95% confidence interval, 1.04 to 37.05; p=0.045).
Two to 10% of all ischaemic strokes are classified as malignant middle cerebral artery (MCA) infarction9), which is one of the most catastrophic forms of ischaemic stroke. The prognosis of malignant MCA infarction is very poor : approximately 70–80% patients die within the first week after stroke onset despite maximal medical treatment3,8,9,27). Although hemicraniectomy has been shown to reduce mortality in malignant MCA infarction, approximately 20–30% patients still die in the acute phase12–14,29,30). Of the conservative measures in malignant MCA infarction, only hypothermia revealed some beneficial effects10). Hypothermia is an effective therapy to reduce cerebral oedema and intracranial pressure (ICP)17,22). The combined treatment of hypothermia and hemicraniectomy could further reduce mortality in malignant MCA infarction. However, there is some hesitation to the clinical application of hypothermia after hemicraniectomy due to an increased risk of complications, such as infection, coagulopathy, and cardiovascular problems. A therapeutic hypothermia strategy has been recently used in our centre as a last rescue treatment when the progression of the patients indicate poor prognosis after hemicraniectomy. In this study, we investigated the clinical efficacy and safety of hypothermia treatment after hemicraniectomy in patients with malignant MCA infarction.
The patients were collected from our prospectively maintained stroke registry between January 2010 and February 2016. During this period, a total of 103 patients with malignant MCA infarction underwent decompressive hemicraniectomy in Dong-A University Hospital. Of these patients, 59 patients were treated before implementation of hypothermia as a treatment strategy in November 2012 (early period), and 44 thereafter (late period). From November 2012 to February 2016, 20 patients were treated with hypothermia at 34°C after the hemicraniectomy (hypothermia group). The indication of hypothermia included acute cerebral infarction involving more than two-thirds of MCA territory and a mental status decrease to stupor or worse (a Glasgow coma scale [GCS] score <11) with midline shift >10 mm or a transtentorial herniation sign (a fixed and dilated pupil). We retrospectively collected 27 patients, as the control group, who had underwent hemicraniectomy alone and simultaneously met the inclusion criteria of hypothermia between January 2010 and October 2012. Our Institutional Review Board approved this study for retrospective analysis.
In all patients in the hypothermia group, hypothermia was induced immediately after hemicraniectomy or after the decision of hypothermia during postoperative care. Hypothermia was achieved by a servo-controlled water-filled thermal blanket set to maintain the rectal temperature at 34°C (Blanketrol III, Cincinnati Sub-Zero, Cincinnati, OH, USA). The duration of hypothermia was determined according to the findings of follow-up CT scans, which were routinely performed every 2 to 3 days, as well as the condition of the patient. Active rewarming was attempted by a rate of 0.2°C/hr. The patients were sedated with midazolam (0.02 to 0.1 mg/kg/hr) and vecuronium (0.8 to 1.4 mcg/kg/min) during hypothermia. The patients were ventilated with continuous mandatory ventilation mode, and pCO2 was maintained between 30 and 40 mmHg. Forced hyperventilation was not used in any case. All patients were positioned at approximately 30° head elevation. Crystalloid/colloid fluids or catecholamine were applied to maintain a mean blood pressure of 90–110 mmHg. Mannitol (0.9 to 1.5 g/kg/day) was administered every 4 hours to all the patients in both groups during the first 7 days. Fluid homeostasis was maintained by confirming fluid intake and output every hour, aimed at a central venous pressure between 8 and 12 cm H2O. Serum blood glucose was maintained between 100 and 200 mg/dL by insulin administration if necessary.
Hypothermia-related complications, such as pneumonia, sepsis, coagulation disorders, postoperative bleeding (haemorrhagic transformation, epidural haematoma, and subdural haematoma), cardiac arrhythmia, bradycardia (<40 beats/min), and arterial hypotension (mean arterial blood pressure <80 mmHg) were investigated. A complete blood count, coagulation parameters, and serum levels of liver and pancreas enzymes, urea and creatinine were determined daily, and electrolytes and blood gas analysis were determined every 6 hours. In addition, a chest X-ray was performed daily.
In the hypothermia group and control group, decompressive hemicraniectomy was performed in the same manner by two neurosurgeons (H.S and J.H) during this study as described elsewhere7). Briefly, a bone flap with a diameter of approximately 14 cm, including the frontal, parietal, temporal and partial occipital squama was removed. The dura was tacked at the craniotomy margin to prevent epidural haematoma. The dura was then widely opened for sufficient decompression, and an autologous or artificial dural patch was placed in the incision. The bone flap was stored under sterile conditions and routinely reimplanted after 3 months.
Clinical assessment was based on the GCS scores and National Institute of Health Stroke Scale (NIHSS) scores, which were investigated immediately after admission and before hemicraniectomy. The extent of cerebral infarction and the degree of midline shift was evaluated on the basis of the follow-up computed tomography (CT) scans within 7 days after stroke onset. The following clinical and radiological data were compared between the two groups : sex, age, infarct location, preoperative infarct volume, preoperative GCS and NIHSS scores, the maximal degree of midline shift on the follow-up CT scan within 7 days from stroke onset, the time interval between symptom onset and hemicraniectomy, and the duration of stay in the intensive care unit of survivors. Primary outcome was defined as either death or survival within the hospital. Assessment of mortality was differentiated into death caused by brain herniation and death as a consequence of treatment-related problems (i.e., pneumonia, sepsis, arrhythmia, cardiac failure, or coagulopathy) or any intra- or perioperative complications (i.e., intraoperative bleeding, postoperative intracerebral haemorrhage, subdural or epidural haemorrhage, or infection of the wound).
Statistical analysis was performed using SPSS software version 21.0 (IBM Corp., Armonk, NY, USA). The chi-squared test, Student t-test, and Mann-Whitney U tests were used as appropriate for comparisons of baseline demographics between the hypothermia and control groups. Normally distributed variables were expressed as the mean±standard deviation, and non-normally distributed variables were expressed as the median and interquartile range (IQR). Logistic regression analysis was performed to calculate the independent contributions of variables for survival. Statistical significance was established at p<0.05.
The target temperature of 34°C was reached within 5±1 hours (range, 3 to 12 hours). Hypothermia was maintained for 4±2 days (range, 1 to 7 days). Of the 20 patients in the hypothermia group, 11 patients were induced with hypothermia immediately after hemicraniectomy. Nine patients of them were induced with hypothermia after the decision of hypothermia during postoperative care, and the time interval between hemicraniectomy and induction of hypothermia was 2.8±1.6 days. Seven patients (35%) showed a transtentorial herniation sign (a fixed and dilated pupil) before hypothermia among 20 patients in the hypothermia group.
This study included 47 patients who met the inclusion criteria of hypothermia after decompressive craniectomy : there were 12 males and eight females in the hypothermia group, and there were 17 males and 10 females in the control group (p=0.836). There are no significant differences between the hypothermia group and control group with regard to age (62±10 years and 59±11 years, p=0.241), preoperative infarct volume (216±63 mL and 218±59 mL, p=0.923), GCS score (8±1 and 8±1, p=0.401) and NIHSS score (18±4 and 17±3, p=0.627) before surgery, maximal degree of midline shift (15±3 mm and 14±2 mm, p=0.540), and time interval between symptom onset and hemicraniectomy (29 hours [IQR, 18–44] and 23 hours [IQR, 14–42], p=0.684) (Table 1).
There was no statistically significant difference of in-hospital mortality between the hypothermia group and the control group, but the mortality of hypothermia group tended to be lower than that of the control group (15.0% vs. 40.7%, p=0.056). In the hypothermia group, the causes of death were transtentorial herniation in two patients and cardiac failure in one patient. In the control group, the causes of death were transtentorial herniation in seven patients, pneumonia in two patients, pulmonary thromboembolism in one patient, and acute kidney injury in one patient. Hypothermia was identified as the strongest factor that affected survival in the multivariable analysis (odds ratio [OR], 6.21; 95% confidence interval [CI], 1.04 to 37.05; p=0.045) (Table 2). Of the 20 patients in the hypothermia group, 18 patients were followed at one year and five patients (27.8%) died within one year, and 25 patients were followed at one year among 27 patients in the control group and 13 patients (52.0%) died within one year (p=0.112). Functional outcomes after 12 months were not statistically different between both groups (p=0.357).
The side effects of hypothermia included two patients with rrhythmia, one patient with sepsis, one patient with pneumonia, and one patient with hypotension; three cases of hypothermia were discontinued due to these side effects (one patient with sepsis, one patient with hypotension, and one patient with bradycardia). Of all the adverse events during the stay in the intensive care unit, pneumonia was the most common adverse event in the hypothermia group (n=5, 25%), but there were no significant differences compared with the control group (n=5, 18.5%, p=0.723). The duration of stay in the intensive care unit did not differ significantly between the hypothermia group and control group (16 days [IQR, 13–22] and 18 days [IQR, 13–23], p=0.914). There were no significant differences in the incidence of haemorrhagic transformation after hemicraniectomy between the hypothermia group (n=7, 35.0%) and control group (n=7, 25.9%; p=0.501), and only one patient in the control group underwent revision surgery for removal of intracerebral haemorrhage in the basal ganglia. Two patients in the hypothermia group revealed epidural haematoma (EDH) on the follow-up CT during hypothermia, and three patients in the control group showed EDH after hemicraniectomy, but they did not require further revision surgery for haematoma removal.
A 54-year-old male presented with left-sided hemiparesis and dysarthria (NIHSS 7) at admission. The magnetic resonance image (MRI) revealed a right-sided MCA infarction (Fig. 1). He underwent endovascular treatment, but its recanalisation failed due to complete occlusion of right cervical internal carotid artery. One day after admission, his neurological symptoms worsened (NIHSS 15) and he underwent decompressive hemicraniectomy. Four days after hemicraniectomy, his mentality worsened to stupor and follow-up CT revealed a marked midline shift at that time. He was immediately cooled to 34°C and hypothermia maintained for four days. The patient gradually improved after hypothermia without any serious adverse events and was transferred to the rehabilitation department. The patient underwent cranioplasty three months after hemicraniectomy, and he could walk without assistance one year later.
A 64-year-old male (left handed) presented to the emergency room with right-sided hemiparesis and aphasia one hour after symptom onset (NIHSS 18). The MRI revealed a right-sided malignant MCA infarction (Fig. 2). One day after admission, his mentality decreased to a drowsy state and the follow-up CT showed an increase in brain swelling with haemorrhagic transformation. The patient underwent decompressive hemicraniectomy. One day after hemicraniectomy, his mentality decreased to a stupor state and the follow-up CT revealed an increase in brain swelling with an increase in haemorrhagic transformation. Hypothermia was immediately induced with 34°C and maintained for seven days. After hypothermia, the patient gradually improved without serious adverse events. He underwent cranioplasty three months later, and he could walk without assistance 10 months after the event.
Although hemicraniectomy clearly reduces the mortality rate in patients with malignant MCA infarction, approximately 20–30% patients still die in the acute phase12–14,29,30). We propose that there is still potential for a decrease in the mortality rate in the treatment for patients with malignant MCA infarction. Therapeutic hypothermia is used for the neuroprotection or prevention of cerebral oedema in acute cerebral infarction. Combined therapy of hemicraniectomy and hypothermia could further reduce the mortality rate, although there are rare studies in the literature. There has been only one study about efficacy of hypothermia combined with hemicraniectomy in malignant MCA infarction, in where Els et al.7) reported that combined therapy with hemicraniectomy and mild hypothermia at 35°C for 48 hours improved functional outcome compared with hemicraniectomy alone. However, in their study, there were no significant differences in the overall mortality rate between the combined therapy group and hemicraniectomy alone group7), potentially due to several reasons, including small sample size and the inclusion criteria of hypothermia, that is, “all patients diagnosed with malignant MCA infarction”. In this study, hypothermia contributed to the decrease in mortality rate on the basis of the logistic regression analysis (OR, 6.21; 95% CI, 1.04 to 37.05; p=0.045). We believe that the main reason of the result difference in mortality compared with Els et al.7)’s study is that this study included selected patients whose mental status decrease to stupor or worse with midline shift >10 mm or a transtentorial herniation sign (a fixed and dilated pupil), which means that this study only included patients implying poor prognosis. Furthermore, the target temperature in our study was lower (34°C) and the duration of hypothermia was longer when comparing with Els et al.7)’s study. Hypothermia strategies, such as target temperature and duration of hypothermia, can differ according to the purpose of the hypothermia treatment, such as neuroprotection or reduction of cerebral oedema. Most hypothermia studies investigated the neuroprotective effects in acute cerebral infarction5,11,15,18–20), but there have been rare clinical reports regarding the reduction in cerebral oedema after malignant cerebral infarction7,24,25).
Regarding the duration of hypothermia, animal studies have reported greater benefits with a longer duration of hypothermia in acute cerebral infarction4,34), but the optimal duration of hypothermia is still unknown in clinical practice. In many hypothermia studies for neuroprotection in acute cerebral infarction, most patients were treated with hypothermia within three days5,11,15,18–20). However, in the case of malignant MCA infarction, we propose that the duration of hypothermia will be longer than three days because the maximum cerebral oedema in malignant MCA infarction is usually present between the 2nd and 5th day after symptom onset and reaches a maximum on the 4th day in the majority of patients8,26,27). Severe cerebral oedemas of patients in the hypothermia group in this study were usually prolonged to five days after stroke onset, and some patients showed severe cerebral oedema seven days after stroke onset. In this study, we tried to maintain hypothermia longer than three days with a maximum duration of hypothermia of seven days. The duration of hypothermia was determined by findings obtained from follow-up CT scans and the condition of the patient.
Different levels of therapeutic hypothermia are defined : mild (>32°C), moderate (28–32°C), deep (20–28°C), profound (5–20°C), and ultraprofound (<5°C)28). Although a target temperature of 32–35°C has been used in the majority of clinical trials of hypothermia in acute cerebral infarction5,7,11,15,18–20,24,25), the optimal target temperature for neuroprotection or reduction of cerebral oedema is unknown. Therapeutic hypothermia has been widely studied for neuroprotection in patients who have been successfully resuscitated after cardiac arrest, and it is recently recommended for cardiac arrest patients in international resuscitation guidelines. A previous randomized controlled trial compared a target temperature of 33°C vs. 36°C in patients resuscitated after cardiac arrest, and reported that there were no significant differences between the two groups in the overall mortality rate and functional outcome at 6 months21). However, we propose that the optimal target temperature for a reduction in cerebral oedema in acute cerebral infarction is different with that for neuroprotection after cardiac arrest. Kollmar et al.16) reported a U-shaped curve of effectiveness of hypothermia in acute cerebral infarction with 34°C, which yielded the best result. However, further research is required to evaluate the optimal target temperature and duration of hypothermia for patients with malignant MCA infarction.
Similar to the uncertainty regarding the duration and target temperature of hypothermia, the optimal timing of starting hypothermia is unknown. Animal experiments and previous studies suggest that rapid initiation of hypothermia yielded greater benefits31,32). However, in clinical practice, some patients with malignant MCA infarction showed a benign and stable course after early hemicraniectomy, and these patients did not require further invasive therapy, such as hypothermia. In this study, we started hypothermia as a last rescue treatment only when patients with malignant MCA infarction were expected to have poor prognosis after hemicraniectomy. Initiating hypothermia as soon as possible after stroke onset may potentially provide a better functional outcome by reducing final infarct size. However, applying hypothermia to patients with malignant MCA infarction should be carefully considered because hypothermia-related complications occur such as infection by immunosuppression, postoperative bleeding by coagulopathy, and other medical problems.
In this study, infection problems, such as pneumonia and sepsis were more common in the hypothermia group, though there were no statistically significant differences between the two groups. Although a Cochrane Review on hypothermia for acute stroke reported a non-significant increase in the occurrence of infections related to hypothermia6), an ICTuS-L study showed a statistical increase in pneumonia in the hypothermia group compared with the normothermic group11). Cardiovascular problems, including bradycardia, hypotension, and arrhythmia occurred during hypothermia in this study, which was also reported in previous studies1,2). These events may be harmful for maintaining appropriate cerebral blood flow in acute cerebral infarction. Reduced platelet function and coagulation enzyme activity during hypothermia has also been reported in previous studies, which can potentially increase the risk of haemorrhagic transformation and postoperative bleeding such as epidural or subdural haematoma after hemicraniectomy. In this study, there were no significant differences in the problems of haemorrhagic transformation and postoperative bleeding between the two groups. We think that meticulous control of bleeding during surgery and appropriate placement of the haemovac catheter are important.
The main limitations of this study were its retrospective nature and non-contemporaneous control group. The baseline characteristics of both groups were not significantly different, but the selection bias can be in this study. However, hemicraniectomy and postoperative care were performed by the same neurosurgeons in a similar manner during this study. This study enrolled patients who underwent hemicraniectomy, which indicated that most patients did not have severe premorbidity or medical problems prior to hemicraniectomy. Furthermore, the overall mortality of patients with malignant MCA infarction decreased from 28.8% (early period) to 18.8% (later period) after implementation of the hypothermia strategy, and we propose that hypothermia is one important reason contributing to the decrease mortality in malignant MCA infarction. Another limitation of this study is that ICP monitoring was not used in this study. Current guideline suggests that routine ICP monitoring is not indicated in malignant MCA infarction33), and there does not appear to be any value of ICP monitoring in patients with malignant MCA infarction23). In patients with malignant MCA infarction, pupillary abnormalities and severe brain stem compression may be present despite normal ICP values23), and we think that serial follow-up CT scans and checking pupillary responses during hypothermia are important when deciding how long to undergo hypothermia. Another important limitation of this study was the small sample size, which was insufficient to reveal any conclusive benefits of hypothermia. Large randomized controlled trials are required to demonstrate its benefits in clinical practice.
In this study, hypothermia treatment contributed to the survival of patients with malignant MCA infarction when the patients showed neurological worsening due to the progression of cerebral oedema despite hemicraniectomy. Hypothermia after hemicraniectomy did not significantly increase the hypothermia related complications, such as infection, coagulopathy, and cardiovascular problems. This study suggests that hypothermia is a viable therapeutic option when a patient with malignant MCA infarction is expected to have poor prognosis after hemicraniectomy. However, further research is required to determine the optimal target temperature, timing and duration of hypothermia for patients with malignant MCA infarction.
References
1. Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 31:2041–2051. 2003.

2. Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med. 30:146–153. 1997.

3. Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 24:620–623. 1998.

4. Clark DL, Penner M, Orellana-Jordan IM, Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 212:386–392. 2008.

5. De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for acute ischemic brain damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 63:312–317. 2004.

6. Den Hertog HM, van der Worp HB, Tseng MC, Dippel DW. Cooling therapy for acute stroke. Cochrane Database Syst Rev. (1):CD001247. 2009.

7. Els T, Oehm E, Voigt S, Klisch J, Hetzel A, Kassubek J. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis. 21:79–85. 2006.

8. Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology. 45:1286–1290. 1995.

9. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 53:309–315. 1996.

10. Heiss WD. Malignant MCA Infarction: pathophysiology and imaging for early diagnosis and management decisions. Cerebrovasc Dis. 41:1–7. 2016.

11. Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 41:2265–2270. 2010.

12. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, et al. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 8:326–333. 2009.

13. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke. 38:2518–2525. 2007.

14. Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 370:1091–1100. 2014.

15. Kammersgaard LP, Rasmussen BH, Jorgensen HS, Reith J, Weber U, Olsen TS. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: a case-control study: the copenhagen stroke study. Stroke. 31:2251–2256. 2000.

16. Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 38:1585–1589. 2007.

17. Kollmar R, Schäbitz WR, Heiland S, Georgiadis D, Schellinger PD, Bardutzky J, et al. Neuroprotective effect of delayed moderate hypothermia after focal cerebral ischemia: an MRI study. Stroke. 33:1899–1904. 2002.

18. Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, et al. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 32:1847–1854. 2001.
19. Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, et al. Intravascular cooling in the treatment of stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 14:107–114. 2005.

20. Martin-Schild S, Hallevi H, Shaltoni H, Barreto AD, Gonzales NR, Aronowski J, et al. Combined neuroprotective modalities coupled with thrombolysis in acute ischemic stroke: a pilot study of caffeinol and mild hypothermia. J Stroke Cerebrovasc Dis. 18:86–96. 2009.

21. Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 369:2197–2206. 2013.

22. Park CK, Jun SS, Kim MC, Kang JK. Effects of systemic hypothermia and selective brain cooling on ischemic brain damage and swelling. Acta Neurochir Suppl. 71:225–228. 1998.

23. Poca MA, Benejam B, Sahuquillo J, Riveiro M, Frascheri L, Merino MA, et al. Monitoring intracranial pressure in patients with malignant middle cerebral artery infarction: is it useful? J Neurosurg. 112:648–657. 2010.

24. Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 32:2033–2035. 2001.

25. Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 29:2461–2466. 1998.

26. Shaw CM, Alvord EC Jr, Berry RG. Swelling of the brain following ischemic infarction with arterial occlusion. Arch Neurol. 1:161–177. 1959.

27. Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 15:492–496. 1984.

28. Tahir RA, Pabaney AH. Therapeutic hypothermia and ischemic stroke: a literature review. Surg Neurol Int. 7(Suppl 14):S381–S386. 2016.

29. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 6:215–222. 2007.

30. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 38:2506–2517. 2007.

31. van der Worp HB, Macleod MR, Kollmar R; European Stroke Research Network for Hypothermia (EuroHYP). Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab. 30:1079–1093. 2010.

32. van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 130(Pt 12):3063–3074. 2007.

33. Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 45:1222–1238. 2014.

Fig. 1
A : Apparent diffusion coefficient map of magnetic resonance (MR) image demonstrating an acute cerebral infarction in the right MCA territory. B : MR angiography showing occlusions of right internal carotid artery and MCA. C : One day after admission, the patient underwent decompressive hemicraniectomy because his neurological symptoms worsened. D : Four days after hemicraniectomy, his mentality worsened to stupor showing a marked midline shift on the follow-up CT. E : Four days after hypothermia treatment, follow-up CT revealing an improved state of cerebral oedema. F : The patient underwent cranioplasty 3 months after hemicraniectomy, follow-up CT. MCA : middle cerebral artery, CT : computed tomography.
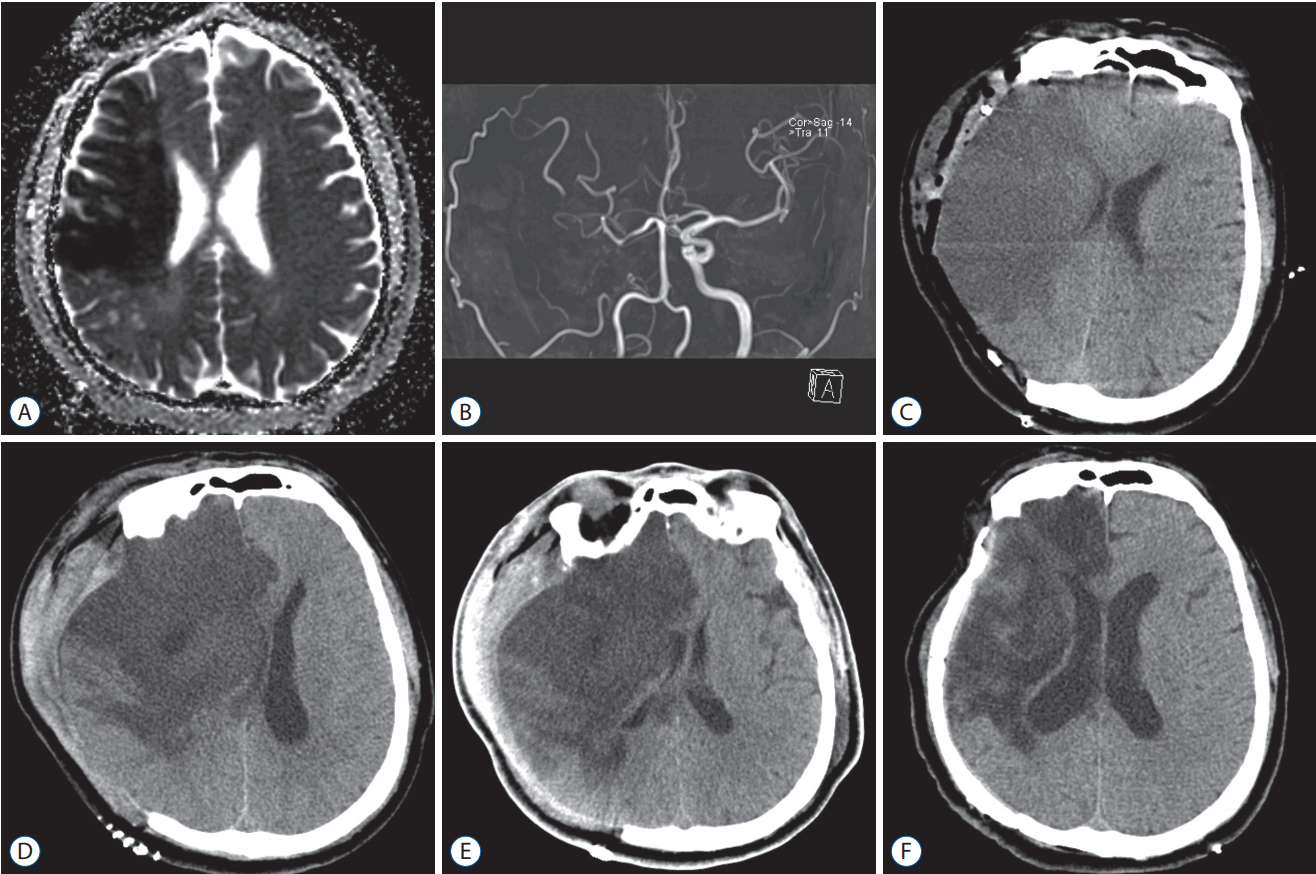
Fig. 2
A : Magnetic resonance (MR) image showing a large acute infarction in the right MCA territory at admission. B : MR angiography demonstrating an occlusion of right internal carotid artery terminus portion. C : One day after admission, the patient underwent decompressive hemicraniectomy due to an increase of cerebral oedema with haemorrhagic transformation. D : One day after hemicraniectomy, his mentality worsened from drowsy to stupor with an increase in cerebral oedema and haemorrhagic transformation on the follow-up CT. E : Seven days after hypothermia treatment, the follow-up CT showed an improved state of cerebral oedema. F : Ten months later, the patient could walk alone without assistance, although the follow-up MR image revealed a large porencephaly and cerebromalacia in the right MCA territory. MCA : middle cerebral artery, CT : computed tomography.
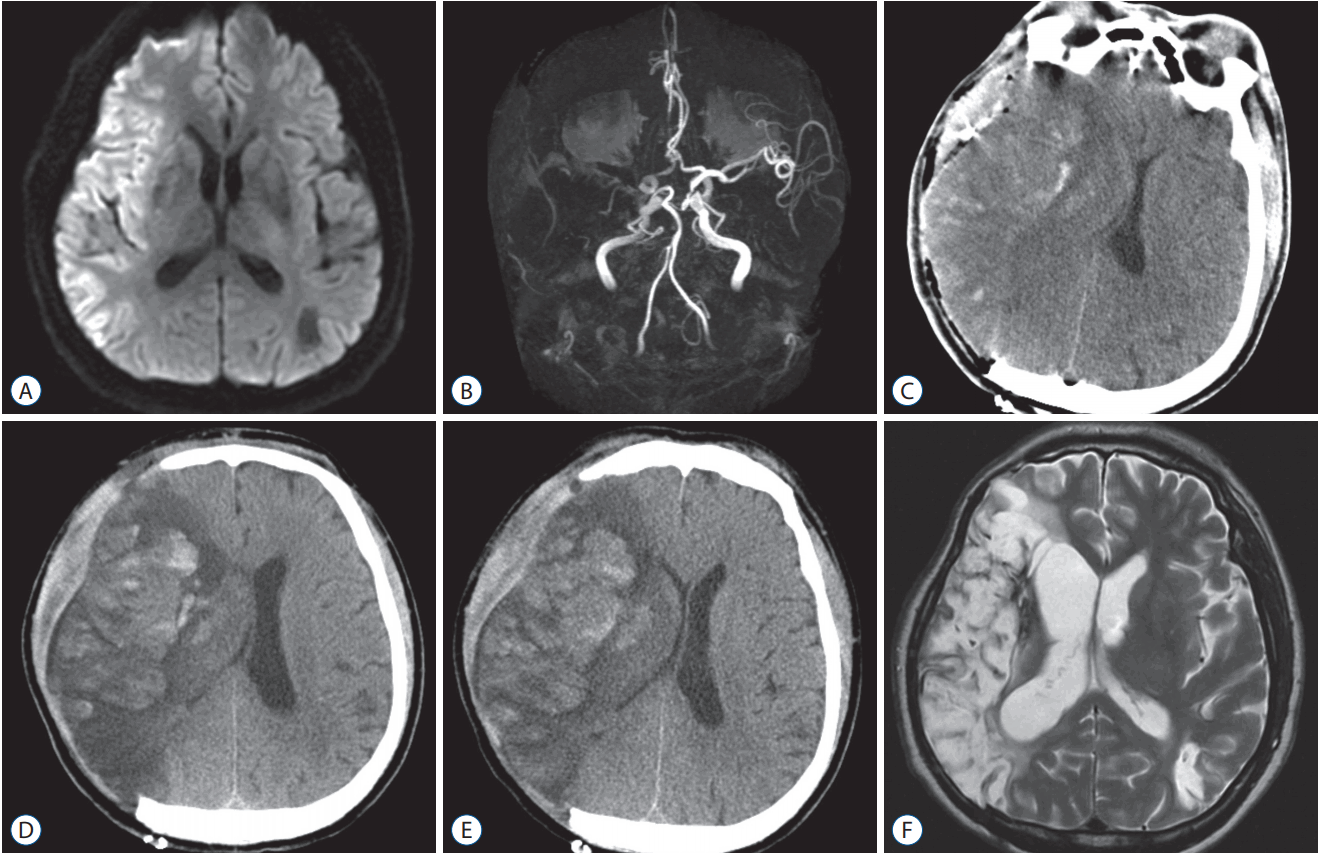
Table 1
Baseline characteristics of the hypothermia and control groups
Table 2
Multivariate logistic analysis for independent factors associated with survival




 Citation
Citation Print
Print


 XML Download
XML Download