Abstract
Objective
The objectives of this study were to evaluate the immediate and long-term efficacy and safety of coil embolization for large or giant aneurysms.
Methods
One hundred and fifty large or giant aneurysm cases treated with endovascular coil embolization between January 2005 and February 2014 at a single institute were included in this study. Medical records and imaging findings were reviewed. Statistical analysis was performed to evaluate prognostic factors associated with major recurrence (major recanalization or rupture) and delayed thromboembolism after selective coil embolization.
Results
Procedure-related symptomatic complications occurred in five (3.3%) patients. The mean clinical and radiological follow-up periods were 38 months (range, 2–110) and 26 months (range, 6–108), respectively. During the follow-up period, the estimated recurrence rate was 4.6% per year. Multivariate analysis using Cox regression showed the degree of occlusion to be the only factor associated with recurrence (p=0.008, hazard ratio 3.15, 95% confidence interval 1.34–7.41). The patient’s history of rupture in addition to the size and location of the aneurysm were not associated with recurrence in this study. Delayed infarction occurred in eight cases, and all were incompletely occluded.
Conclusion
Although immediate postprocedural safety profiles were reasonable, longterm results showed recanalization and thromboembolic events to occur continuously, especially in patients with incomplete occlusion. In addition, incomplete occlusion was associated with delayed thromboembolic complications. Patients with incomplete occlusions should be followed carefully for delayed recurrence or delayed thromboembolic events.
Endovascular coil embolization is a well-established treatment method for intracranial aneurysms18). Recent evidences suggested that endovascular treatment is not inferior to surgical treatment8). The growing use of endovascular treatment has also been fueled by patient preference and the development of new devices1,10).
Despite these advancements, several limitations and major concerns remain. First, procedural rupture and thromboembolic complications are associated with selective coil embolization, and potentially catastrophic complications are possible12,27). Second, endovascular treatments showed inferiority in terms of durability19). Potential predictors of recanalization include subarachnoid hemorrhage, aneurysm size, degree of initial packing, location, and configuration of the aneurysm. However, studies showed variable outcomes regarding the utility of the above factors as predictors4,6,18,26). To the best of our knowledge, only a few studies have explored treatment outcomes of large or giant aneurysms due to a paucity of cases, despite the fact that large size is the most important risk factor for recurrence4,7,22). Thus, prognosis of large or giant aneurysm after selective coil embolization remains unclear. Flow diversion was suggested as a new treatment method for large or giant aneurysms, but immediate and long-term outcomes have not been fully elucidated24). Furthermore, delayed thromboembolic complications in embolized large or giant aneurysms have not been investigated, despite being relatively common12).
In this study, we retrospectively reviewed our data, focusing on recurrence and delayed thromboembolism to identify the actuarial rate of these delayed complications and to identify potential risk factors.
This study was approved by the institutional review board of our institution, and patient consent was waived because of the retrospective study design Between January 2005 and February 2014, 1479 intracranial aneurysms in 1334 consecutive patients were treated at our institution. Inclusion criteria were aneurysms >10 mm in diameter and treatment with selective coil embolization. Exclusion criteria were small aneurysms and aneurysms other than berry aneurysms (n=1302), such as dissecting aneurysms, fusiform aneurysms, and traumatic pseudo aneurysms. We also excluded patients who were treated by parent artery occlusion (n=12) and those who were lost to follow-up within 3 months after treatment (n=15). One hundred and fifty cases from our single-institutional registry were included this study. Medical records, including procedural notes, digital subtracted angiography (DSA), pre and post-procedural imaging, and records noting variables such as age, gender, aneurysm configuration (daughter sac, thrombosed aneurysm, and incorporating artery), location and size of the aneurysm, method of treatment, and occlusion status were reviewed. We also reviewed periprocedural events, such as procedural rupture or thromboembolic events.
Regarding characteristics of aneurysm, an incorporating artery was defined as a complete arterial orifice arising from the aneurismal neck. A thrombosed aneurysm was defined as evidence of intra-aneurysmal thrombus on pre-procedural computed tomography or magnetic resonance imaging. Clinical and radiological follow-up periods were calculated from the time of coil embolization to the latest inpatient or outpatient clinic and to the latest magnetic resonance angiography (MRA) or DSA.
The degree of embolization was assessed by two physicians (J.K.I. and C.J.H.). Embolization was considered to be complete if there was no contrast filling the dome, body, or neck of the aneurysm. A neck remnant was defined as residual filling of the aneurysm neck or part of the neck, and contrast agent present in the body or dome of the aneurysm indicated a residual sac. Recurrence was categorized as either minor or major recurrence. Minor recurrence was defined as any increase in aneurysmal neck filling on follow-up angiography (MRA or DSA) that did not require further treatment. Major recurrence was defined as increased aneurysmal filling requiring further treatment such as coil embolization, surgical clipping, or aneurysms that ruptured. Delayed (>1 month) ischemic stroke at corresponding area of parent vessel of aneurysm or progressive stenosis/occlusion of the parent vessel adjacent to aneurysm were considered delayed thromboembolic events.
The primary outcome of this study was major recurrence, and the secondary outcomes were all recurrence including minor recurrence and delayed thromboembolism.
This study was retrospective in design; therefore, follow-up protocols differed between patients. MRA was main follow imaging protocol. MRA including time-of-flight sequence was performed on a 1.5 T or 3 T system with or without contrast enhancement. The first evaluation after treatment was usually performed at six months after coiling. Follow-up evaluations were performed yearly thereafter. If major recurrence was suspected, a subsequent DSA was performed within a month.
In the cases with stent assisted coil embolization, 100 mg aspirin and 75 mg of clopidogrel were prescribed at least seven days prior to the procedure. For stent-assisted procedures in the setting of subarachnoid hemorrhages, patients were loaded with 300 mg aspirin and clopidogrel immediately after the procedure, with or without intravenous tirofiban. Patients were then maintained on daily doses of 75 mg clopidogrel and 100 mg aspirin for six months, followed by 100 mg of aspirin per day indefinitely.
The decision to treat aneurysms with endovascular therapy was based on agreement between the attending neurosurgeon and the interventional radiologist. The decision was based on aneurysm morphology, aneurysm parent vessel relationship, patient preference, and the patient’s surgical comorbidities. In patients with favorable aneurysm geometries, the aim was to selectively and completely coil the aneurysm while preserving the patency of the parent artery. Depending on operator discretion, wide-neck aneurysms were coiled with stents or with balloon assistance. For unruptured aneurysms, coiling was performed with an initial 50 U/Kg heparin bolus and by maintaining the intraoperative activated clotting time at 1.5–2.5 times the baseline rate. Systemic heparinization was not deployed during coil embolization for ruptured aneurysms. Coils (commercially available bare metal or hydrogel coils) were placed until satisfactory obliteration was achieved or until placement of additional coils was not possible.
We performed statistical analysis with SPSS version 20.0 (SPSS Inc., chicago, IL, USA). We performed simple comparisons using Chi-square tests, Fisher’s exact tests, and Mann-Whitney U tests when applicable. To identify potential risk factors for aneurysm recurrence, we performed the log-rank test for univariate analysis. Actuarial rate was estimated by Kaplan-Meier method. Cox regression analysis was used for multivariate analyses. Parameters with p values <0.1 on univariate analysis were entered into multivariate analysis. Hazard ratio (HR) with 95% confidence intervals (95% CI) were calculated using Cox regression analysis. p<0.05 was considered to be statistically significant.
Among 150 aneurysms, 30 were ruptured and 120 were unruptured. Stent-assisted coil embolization (SAC) was used in 54 cases, balloon-assisted coil embolization was used in 19 cases, and simple coil embolization using one or two microcatheters was used in the remaining cases. The patients’ baseline characteristics are described in Table 1. Immediate complete occlusion was achieved in 27 cases, neck remnants remained in 88 cases, and residual sacs remained in 35 cases. Procedural rupture occurred in three cases, and procedural thrombus formation occurred in 13 cases. Procedure-related morbidity occurred in five patients (1 rupture, 4 thromboembolisms). The median clinical and radiological follow-up periods were 38 months (range, 2–110) and 26 months (range, 6–108), respectively.
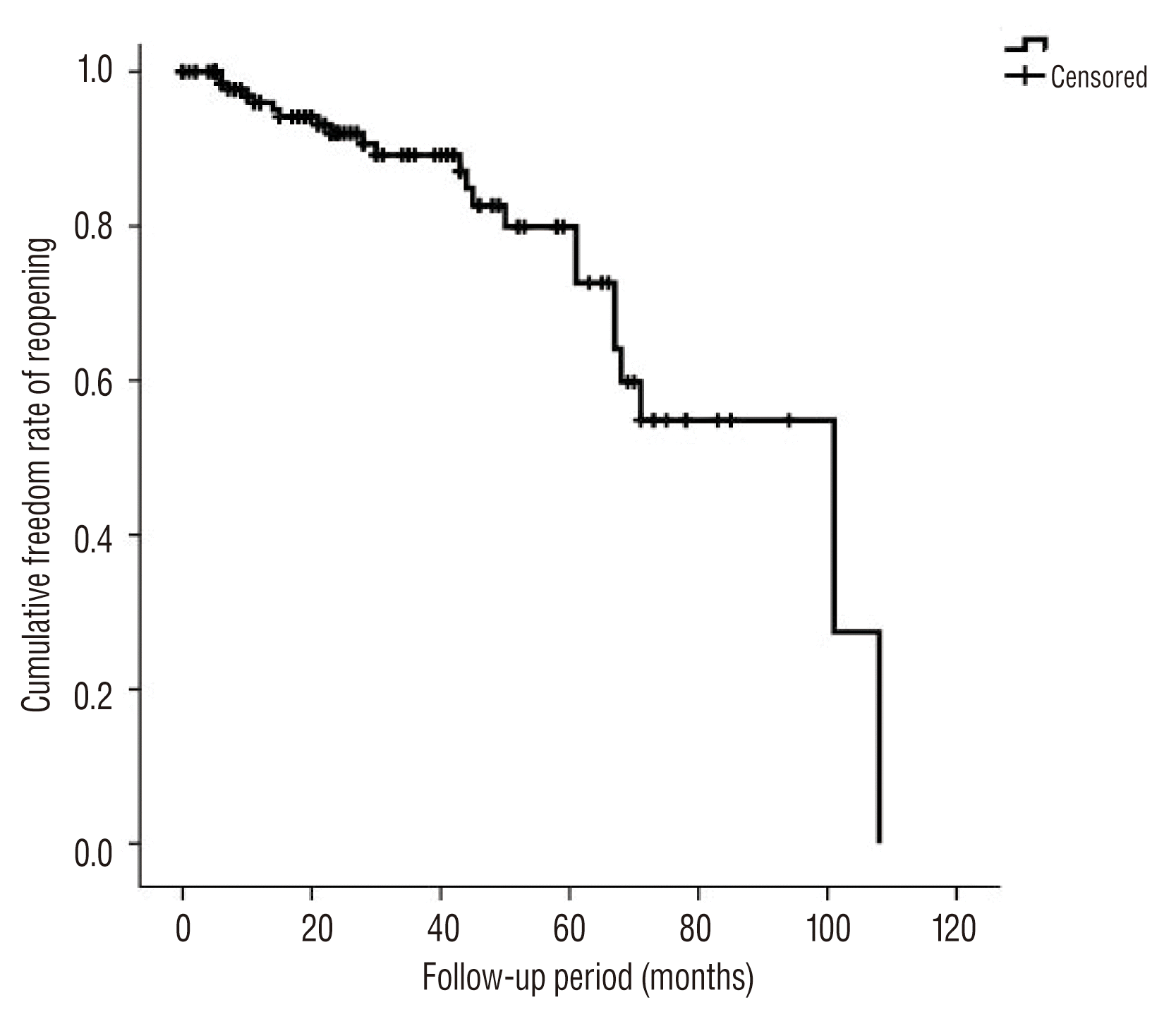
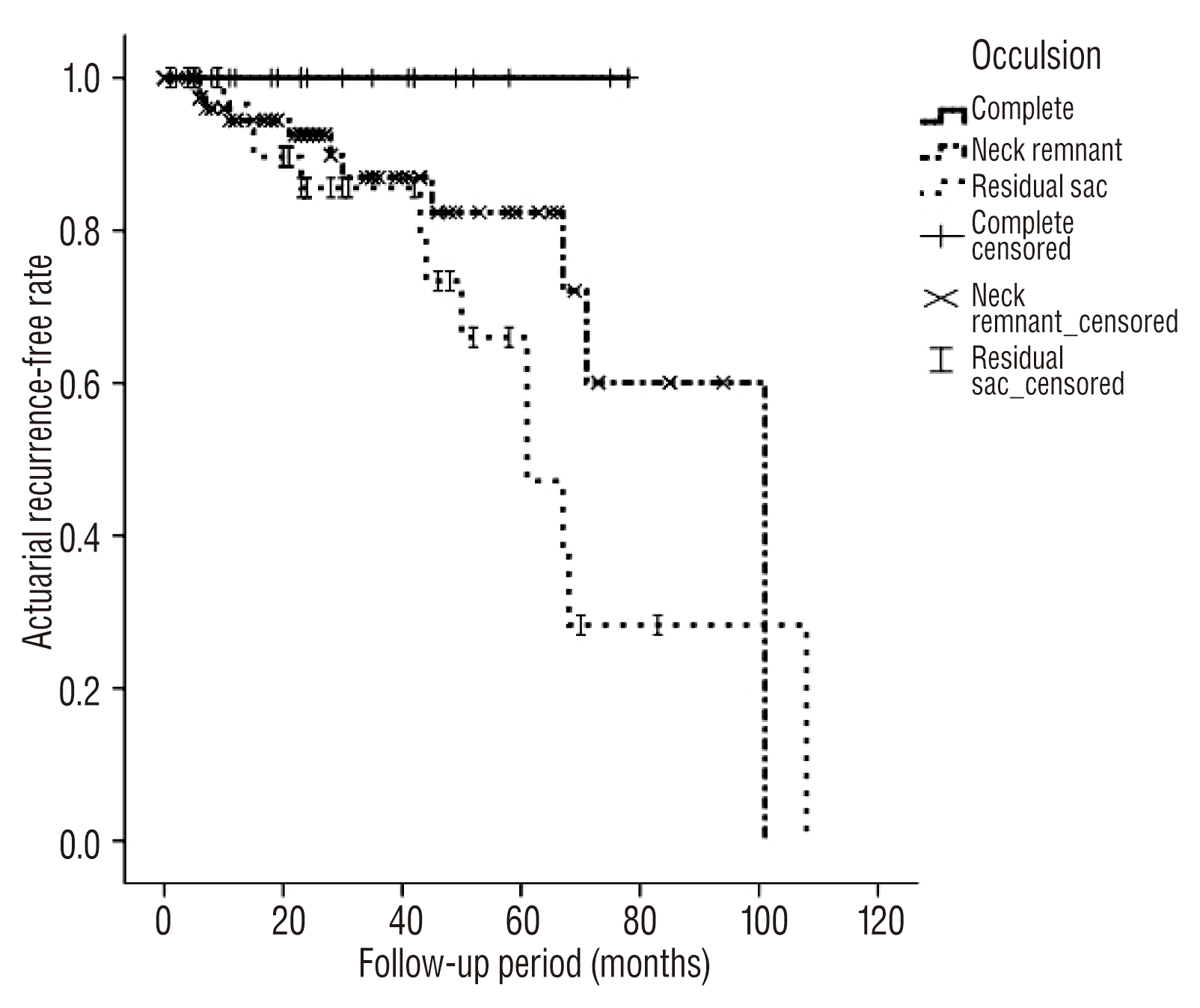
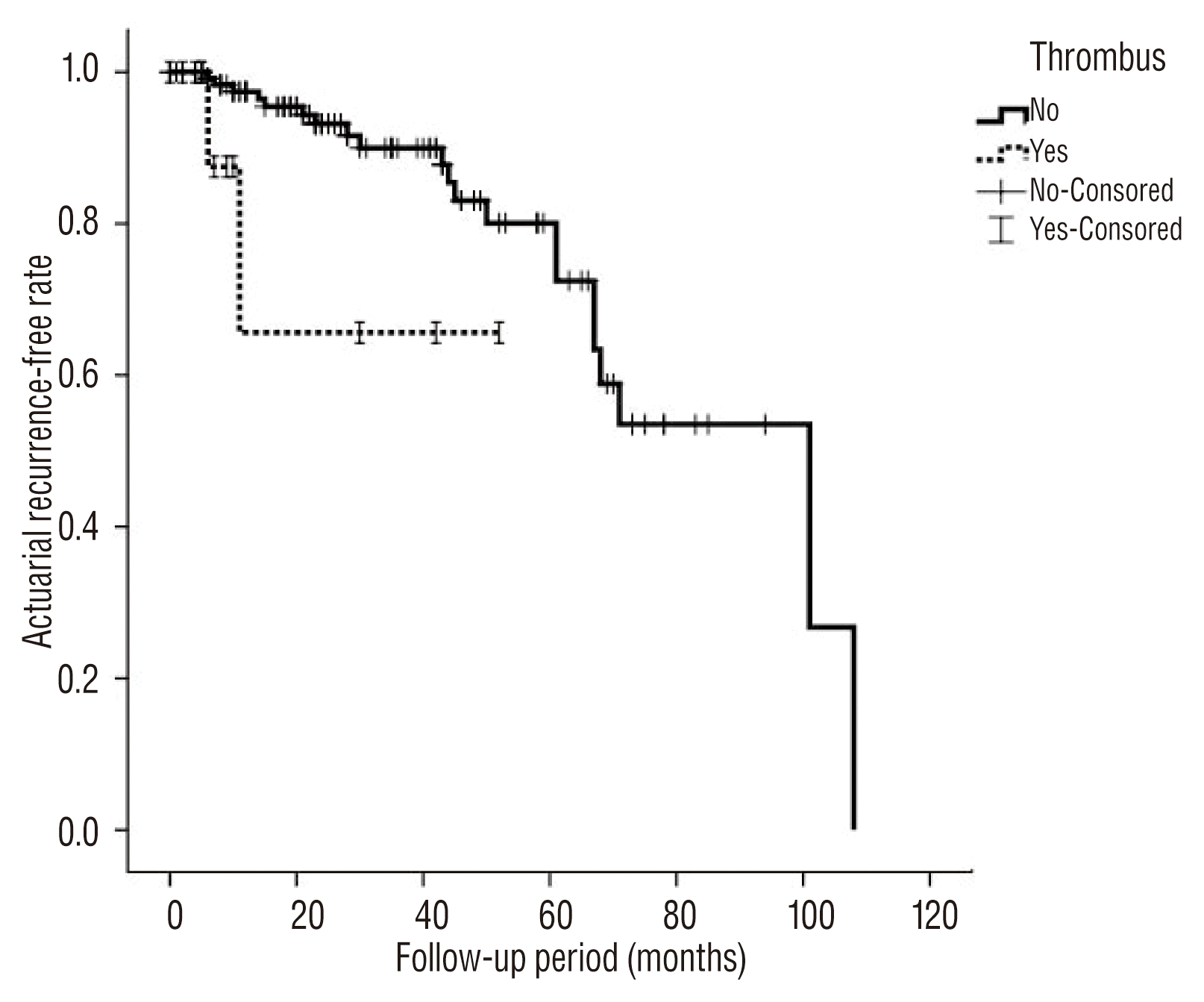
Major recurrence occurred in 23 cases during the follow-up period, including 2 ruptures, 3 symptomatic recurrences, and 18 asymptomatic recurrences. Symptomatic recurrences were associated with mass effect, with one case of stem compression and two cases of cranial nerve palsy. All recurrences were treated with subsequent recoiling. The annual major recurrence rate was 4.6±1.2% per year. The estimated annual rate of major recurrence did not decline during the follow-up period (Fig. 1). Simple comparison test showed that incorporating vessels, daughter sacs, and the degree of occlusion were associated with major recurrence (Table 2). According to univariate and multivariate analysis using log-rank and Cox regression tests, occlusion status was associated with major recurrence (p=0.008, HR 3.15, 95% CI 1.34–7.41) (Table 3, Fig. 2). Thrombosed aneurysms tended to show early recurrence, but this effect was not statistically significant in this data set (Fig. 3). Other variables, such as gender, incorporating vessels, stent usage, daughter sacs, location, size, and rupture were not associated with major recurrence or bleeding (p>0.05). Table 4 showed information of patients with recurrence.
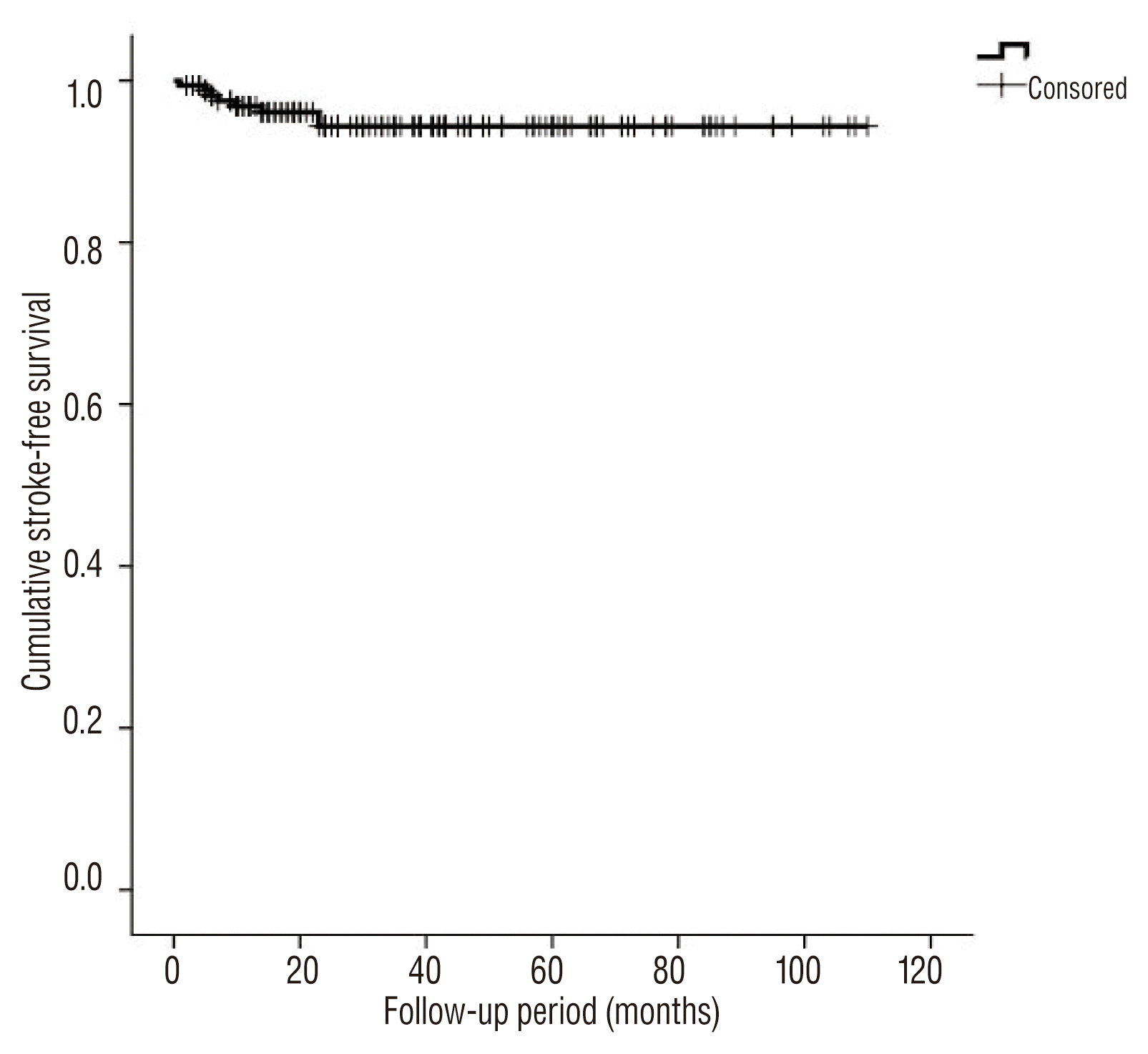
Recurrences, both major and minor, occurred in 51 cases. The actuarial annual overall recurrence rate was estimated to be 8.1±1.3% per year. This rate remained stable at the long-term follow up periods. Delayed thromboembolism occurred in eight patients, and the median time from procedure to delayed thromboembolism was seven months (range, 1–23). All delayed thromboembolic events occurred in the incompletely embolized group, but the differences in delayed thromboembolisms between groups did not show statistically significant (p=0.264, log-rank test). Other variables, including use of stents, gender, incorporating vessels, daughter sacs, aneurysm location, aneurysm size, and rupture status were not associated with delayed thromboembolism in this study (p>0.05). None of the patients showed delayed thromboembolic events after two years (Fig. 4).
Among 54 SAC, 7 were treated with a double-stent overlapping technique with coil embolization to achieve additional flow diversion. Of these seven patients, there were no major recurrance, but two patients had symptomatic thromboembolic events during the clinical follow-up period. However, the association between the double-stent overlapping technique and thromboembolic events was not statistically significant (no stent vs. single stent vs. double stent overlapping, p=0.105, log-rank test).
Among the 35 patients with residual sacs after coil embolization, 28 showed contrast stasis until delayed venous phase at the end of the procedure. Among 28 patients, 24 showed contrast stasis prior to the coil embolization. Ten out of 28 contrast stasis after the coil embolization showed major recurrence during the follow-up period. Newly-developed contrast stasis occurred in four patients with aneurysms, two of which showed major recurrence during follow-up.
Large or giant aneurysms are difficult to treat4,21,22). Although numerous efforts have been made to develop effective treatments for these aneurysms, outcomes remain unsatisfactory. In one recent large case series, a 33% re-treatment rate during 26.3 months of clinical follow-up was demonstrated4,13,23). Our study had a relatively low recurrence rate when compared to this previous study. However, recurrence was not uncommon, and the annual risk of recurrence did not decrease with the length of follow-up.
Previous studies demonstrated that SAC could lower the recurrence rate after coil embolization for cerebral aneurysms16,20). However, our results showed no significant difference between stent use or lack of stent use. Theoretically, stent use could help support the catheter, which could facilitate compact packing and healing2,16). Also, the stent allows for some flow diversion. However, according to our results, single stent could not provide adequate catheter stability and facilitated healing in large or giant aneurysm treatment. Also, using a single stent to divert flow in large or giant aneurysms does not adequately reduce recurrence because of the greater inflow through the large necks of these giant aneurysms. Our subgroup analysis showed that selective coil embolization with double stent overlapping prevented recurrence during the follow-up period. These results suggest that minimal flow diversion with stent overlapping may reduce the risk of aneurysm reopening, while a single stent does not reduce this risk3,14,16). However, our data showed that high rate (28.6%) of delayed thromboembolic events occurred in the double stent overlapping group despite antiplatelet medication. Although our data did not show association between usage of stent and delayed thromboembolic infarction, stent assited coil embolization could be associated with delayed thromboembolism or instent stenosis9). Recent study demonstrated that antiplatelet resistance could be associated with delayed thromboembolism15). Therefore optimal antiplatelet should be used after stent assisted coil embolization. These results should be taken into account in future studies of flow-diverting devices.
This study used visual analysis rather than packing density to evaluate the degree of packing. We thought that packing density could not be applied to certain aneurysms, especially large or giant aneurysms, because a large proportion of aneurysms showed daughter sacs. These daughter sacs made adequate contrast filling of the aneurysms difficult, and filling was not always possible in large or giant aneurysms during 3-dimensional rotatory angiograms. Thus, conventional calculation methods (height×width×depth/2) would be more inaccurate when used with large aneurysms compared to small saccular aneurysms5). In this study, we used visual analysis, which can vary between observers17), but visual analysis by multiple interpreters seemed to be a reliable predictor of recurrence in this study.
Contrast stasis has been reported as a benign angiographic finding after coil embolization11,22). However, this data suggests that contrast stasis is similar to incomplete coil embolization, which is associated with a high risk of recurrence. Although thrombus formation can occur in some patients with contrast stasis, intraluminal thrombus may also be associated with a high risk of recurrence25). In this study, inadequate filling of the aneurysmal sac in large or giant aneurysms was associated with an increased risk of recurrance and delayed stroke. A previous study exploring delayed thromboembolism after stent-assisted coil embolization also showed a high rate of delayed thromboembolism following incomplete aneurysm occlusion12). Aneurysmal necks with loose packing could also be a source of delayed thromboembolism in large or giant aneurysms, regardless of stent use. Therefore, additional coil embolization should be used in large or giant aneurysms if possible.
Previous studies have tended to use simple comparisons with chi-square or Fisher’s exact tests. However, since the risk of aneurysmal reopening is largely influenced by the length of the follow-up period, time should be included as a variable in statistical analyses4). Our data demonstrate that simple comparisons and log rank tests showed differing associations between variables and recurrences. An additional finding in our study is that the annual recurrence rate did not decrease with follow-up time, suggesting that long-term follow-up seems to be essential after coil embolization of large or giant aneurysms. A prospective, long-term follow-up study with large size would be helpful to further assess this issue.
In this study, several limitations should be noted. Major recurrence was defined as any growth of an aneurysm that required additional treatment. This outcome measure is subjective, and inter-observer disagreement may exist. Also, the retrospective design of this study is a major limitation. The retrospective design implies that the study population may be heterogeneous, and follow-up periods and protocols varied. A multicenter, prospective study would be helpful to further explore these questions.
In conclusion, incompletely occluded large or giant aneurysms showed a constant reopening rate (4.6% per year) during follow-up periods (mean, 38.3 months) after selective coil embolization. Therefore, long-term follow-up imaging is essential in order to reduce the risk of delayed rupture. Incompletely occluded large or giant aneurysm was also associated with delayed thromboembolism within two years. A large, prospective, long-term study is needed to further verify the role of endovascular treatment and to help overcome the limitations of endovascular treatment for large or giant aneurysms.
References
1. Bradac O, Hide S, Mendelow DA, Benes V. Aneurysm treatment in Europe 2010: an internet survey. Acta Neurochir (Wien). 154:971–978. discussion 977–978. 2012.

2. Brinjikji W, Kallmes DF, Kadirvel R. Mechanisms of healing in coiled intracranial aneurysms: a review of the literature. AJNR Am J Neuroradiol. 36:1216–1222. 2015.

3. Chalouhi N, Dumont AS, Hasan D, Tjoumakaris S, Gonzalez LF, Starke RM, et al. Is packing density important in stent-assisted coiling? Neurosurgery. 71:381–386. discussion 386–387. 2012.

4. Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Starke RM, Hasan D, et al. Coiling of large and giant aneurysms: complications and long-term results of 334 cases. AJNR Am J Neuroradiol. 35:546–552. 2014.

5. Dengler J, Maldanger N, Bijlenga P, Burkhardt JK, Graewe A, Guhl S, et al. Quantifying unruptured giant intracranial aneurysms by measuring diameter and volume--a comparative analysis of 69 cases. Acta Neurochir (Wien). 157:361–368. discussion 368. 2015.

6. Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 40:e523–e529. 2009.
7. Gao X, Liang G, Li Z, Wei X, Cao P. A single-centre experience and follow-up of patients with endovascular coiling of large and giant intracranial aneurysms with parent artery preservation. J Clin Neurosci. 19:364–369. 2012.

8. Gerlach R, Beck J, Setzer M, Vatter H, Berkefeld J, Du Mesnil de Rochemont R, et al. Treatment related morbidity of unruptured intracranial aneurysms: results of a prospective single centre series with an interdisciplinary approach over a 6 year period (1999–2005). J Neurol Neurosurg Psychiatry. 78:864–871. 2007.

9. Heller R, Calnan DR, Lanfranchi M, Madan N, Malek AM. Incomplete stent apposition in Enterprise stent-mediated coiling of aneurysms: persistence over time and risk of delayed ischemic events. J Neurosurg. 118:1014–1022. 2013.

10. Huang MC, Baaj AA, Downes K, Youssef AS, Sauvageau E, van Loveren HR, et al. Paradoxical trends in the management of unruptured cerebral aneurysms in the United States: analysis of nationwide database over a 10-year period. Stroke. 42:1730–1735. 2011.

11. Hwang G, Jung C, Sheen SH, Park H, Kang HS, Lee SH, et al. Two-year follow-up of contrast stasis within the sac in unruptured aneurysm coil embolization: progressive thrombosis or enlargement? AJNR Am J Neuroradiol. 31:1929–1934. 2010.

12. Hwang G, Kim JG, Song KS, Lee YJ, Villavicencio JB, Suroto NS, et al. Delayed ischemic stroke after stent-assisted coil placement in cerebral aneurysm: characteristics and optimal duration of preventative dual antiplatelet therapy. Radiology. 273:194–201. 2014.

13. Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol. 23:1580–1588. 2002.
14. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 42:2425–2430. 2011.

15. Kim MS, Jo KI, Yeon JY, Kim JS, Kim KH, Jeon P, et al. Safety and efficacy of antiplatelet response assay and drug adjustment in coil embolization: a propensity score analysis. Neuroradiology. 58:1125–1134. 2016.

16. Lawson MF, Newman WC, Chi YY, Mocco JD, Hoh BL. Stent-associated flow remodeling causes further occlusion of incompletely coiled aneurysms. Neurosurgery. 69:598–603. discussion 603–604. 2011.

17. McDonald JS, Carter RE, Layton KF, Mocco J, Madigan JB, Tawk RG, et al. Interobserver variability in retreatment decisions of recurrent and residual aneurysms. AJNR Am J Neuroradiol. 34:1035–1039. 2013.

18. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 8:427–433. 2009.

19. Peluso JP, van Rooij WJ, Sluzewski M, Beute GN. Coiling of basilar tip aneurysms: results in 154 consecutive patients with emphasis on recurrent haemorrhage and re-treatment during mid- and long-term follow-up. J Neurol Neurosurg Psychiatry. 79:706–711. 2008.

20. Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 41:110–115. 2010.
21. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 34:1398–1403. 2003.

22. Raymond J, Klink R, Chagnon M, Barnwell SL, Evans AJ, Mocco J, et al. Patients prone to recurrence after endovascular treatment: periprocedural results of the PRET randomized trial on large and recurrent aneurysms. AJNR Am J Neuroradiol. 35:1667–1676. 2014.

23. Suzuki S, Kurata A, Yamada M, Iwamoto K, Nakahara K, Niki J, et al. Contrast stasis in large and giant internal carotid artery aneurysms as a good prognostic factor for endovascular coil embolization: retrospective study. Neurol Res. 33:832–834. 2011.

24. Turk AS 3rd, Martin RH, Fiorella D, Mocco J, Siddiqui A, Bonafe A. Flow diversion versus traditional endovascular coiling therapy: design of the prospective LARGE aneurysm randomized trial. AJNR Am J Neuroradiol. 35:1341–1345. 2014.

25. van Rooij WJ, Sprengers ME, Sluzewski M, Beute GN. Intracranial aneurysms that repeatedly reopen over time after coiling: imaging characteristics and treatment outcome. Neuroradiology. 49:343–349. 2007.

Table 1
Baseline patient characteristics
Table 2
Characteristics of patients with and without major recurrence
Table 3
Parameters potentially affecting major recurrence
Table 4
Information of the patients with major recurrence




 Citation
Citation Print
Print


 XML Download
XML Download