INTRODUCTION
HISTORY OF ENDOSCOPIC SPINE SURGERY
ENDOSCOPIC LUMBAR SPINE SURGERY
Transforaminal endoscopic lumbar discectomy (TELD)
Contraindications
- Extensive migrated disc and extensive calcification of disc
- At L5–S1 level (particularly in male patients, the patient with long iliac wings)
- More than one level (relative contraindication)
- Spinal canal and foraminal stenosis (relative contraindication)
- Spondylolisthesis
- Recurrent disc herniation (reoperation)
- Nerve root anomalies such as conjugate root
- Cauda equine syndrome
Surgical approach
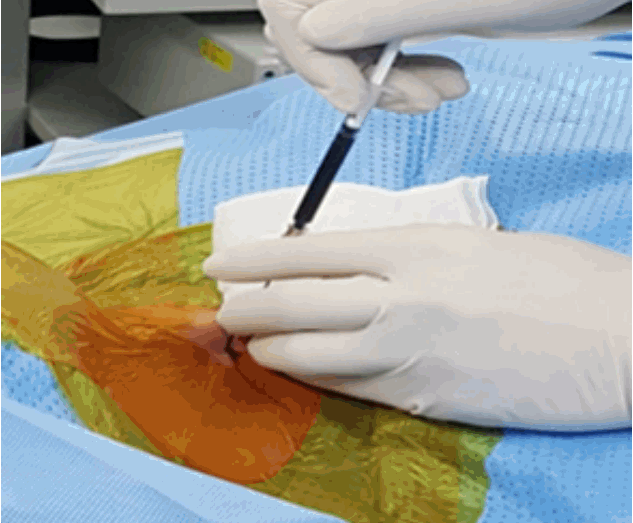
- TELD is performed under local anaesthesia (1% lidocaine) and conscious sedation with midazolam (0.05 mg/kg, 30 mintues before surgery) and fentanyl (0.8 μg/kg, 10 mintues before surgery) in prone or lateral position. Prone position is preferred by most of the surgeons due to better anatomical orientation.
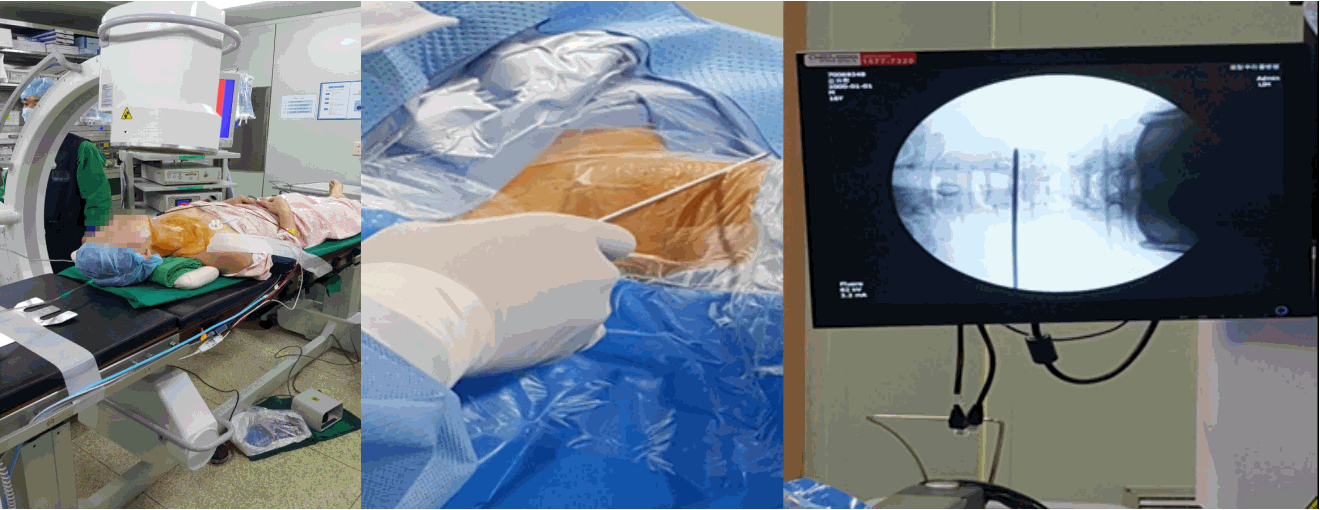
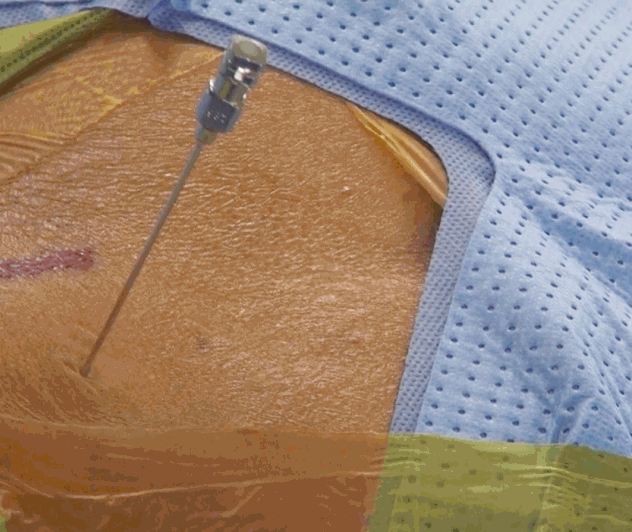
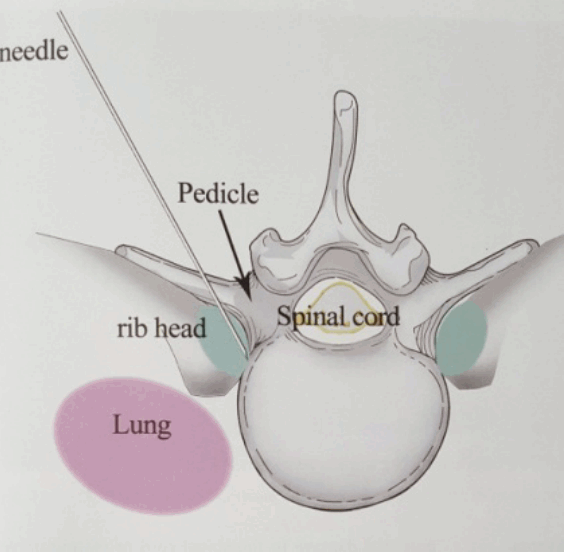
- Skin Entry point for needle insertion is calculated based on pre-operative MRI and computed tomography (CT scan) by measuring distance from midline and needle trajectory aimed to target ruptured fragment without entering peritoneal sac and just to graze the facet.
- Skin entry point is infiltrated with 1% lidocaine then Needle is directed 10 degrees downwards to make 10 degree angle with upper and lower end plates respectively and advanced further till first bony resistance of facet is encountered in C-arm anteroposterior (AP) view, minor adjustment of trajectory can be done by bevel of needle, by keeping it up allows to go more superficially and vice-versa. Now under lateral view of C-arm withdraw needle little bit, elevate it and insert into the foramen by grazing the facet.
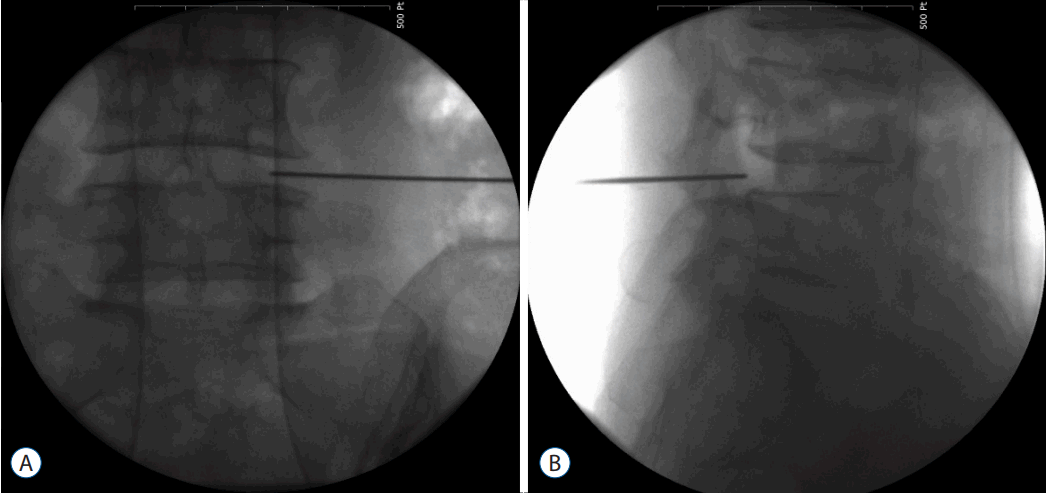
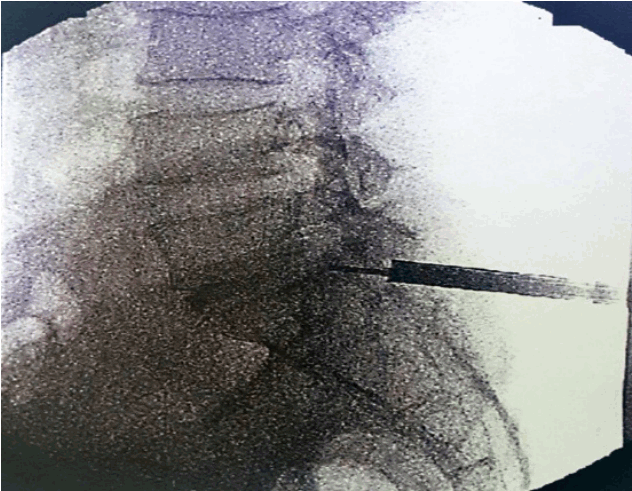
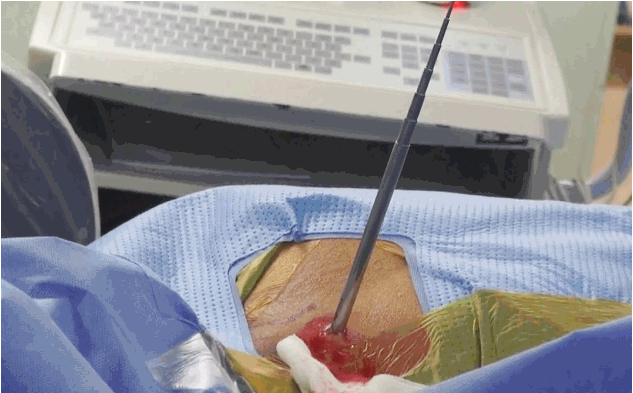
- Most important point is safe and precise landing into the lower part of Kambin’s triangle. Use 6 to 8 mL local anaesthesia into the foramen to make the procedure more comfortable. Site of annular puncture is medial pedicular line in AP view and posterior vertebral line in lateral view in lower lumbar spine as lamina is wider so chance of dural puncture is less and med pedicular line in upper lumbar levels as lamina is narrow so chances of dural puncture is higher. Now pierce the annulus. Haptic feedback is very important (feels like rubber stopper) (Fig. 1).
- Once needle reaches into the centre of the disc in AP view perform a discography by injecting the mixture of 2–3 mL radiopaque dye (Telebrix, Guerbert, France), indigo carmine (Carmine; Korean United Pharma, Seoul, Korea) and normal saline mixed in 2: 1: 2 ratios. Dye helps to identify nuclear fragment during surgery (Fig. 2).
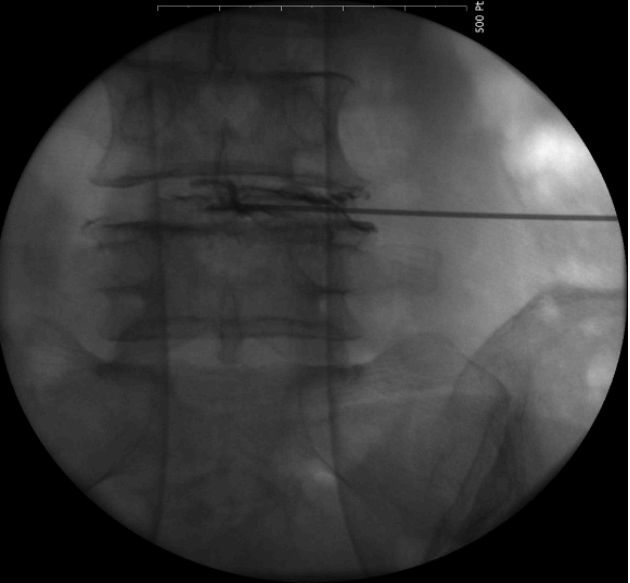
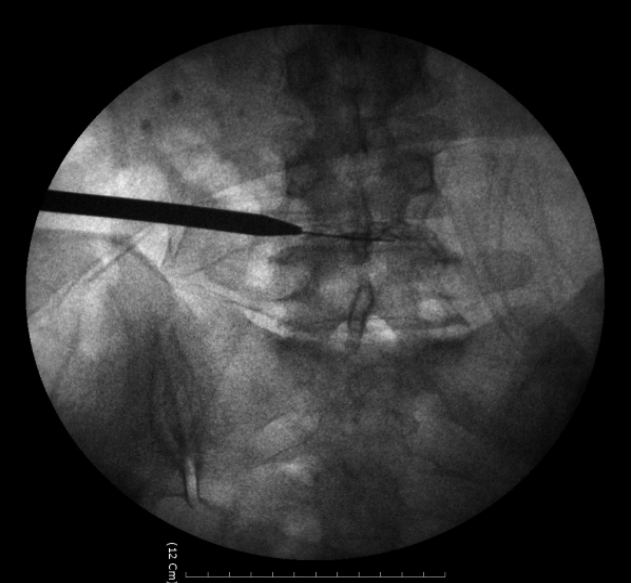
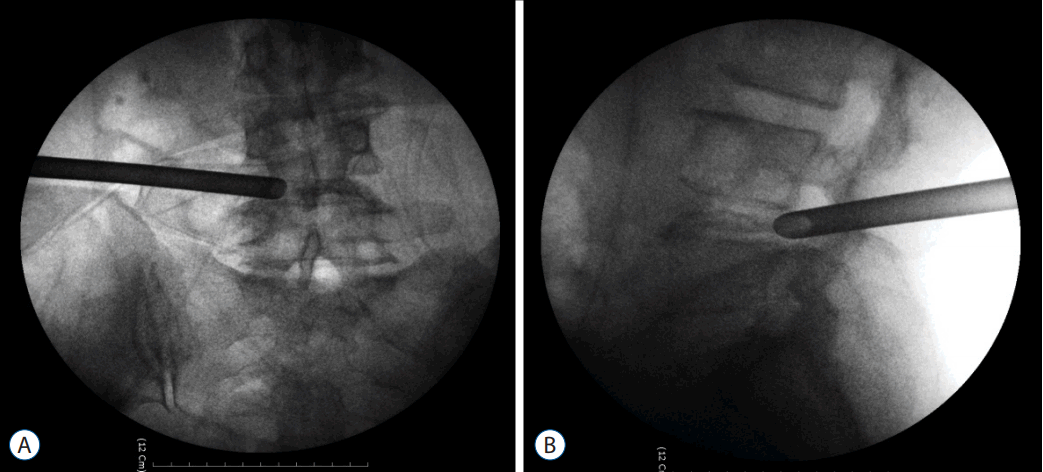
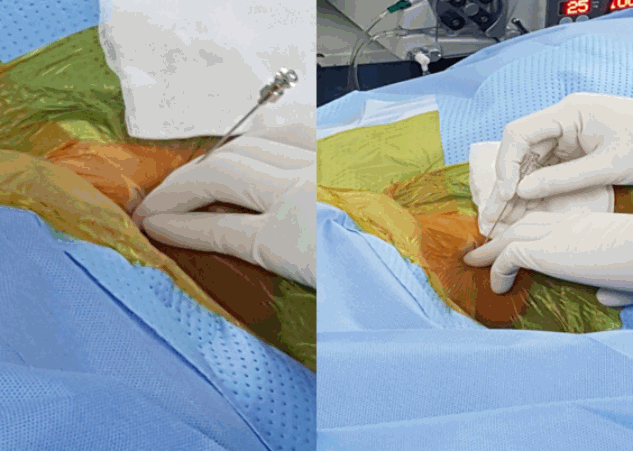
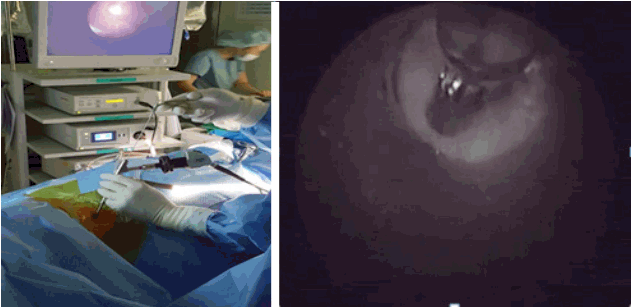
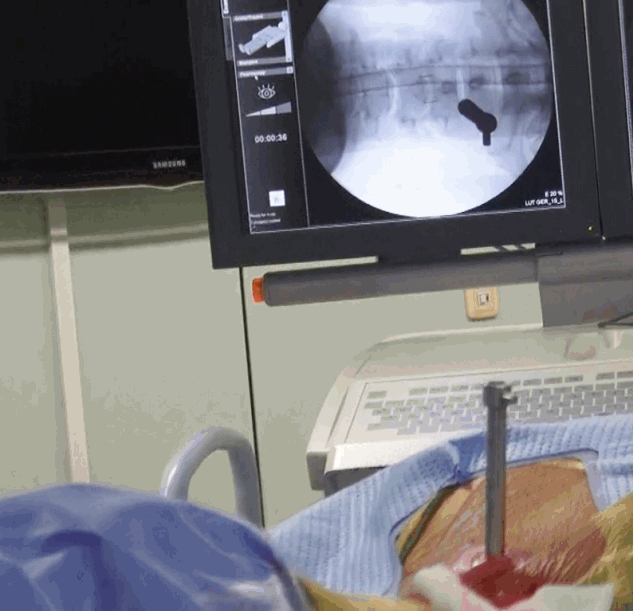
- Replace the needle with guide wire and obturator passed over guide wire till it pierce annulus (Fig. 3). Remove the guide wire and thread the working cannula over obturator with help of mallet & tapper (Fig. 4). Remove the obturator and introduce the endoscope (Spine Doctors, Seoul, Korea) through the working cannula.
- During a procedure watch for any undue pain radiating to the limb which is indicative of compression over exiting root (traversing root protected by facet) and change the needle trajectory accordingly.
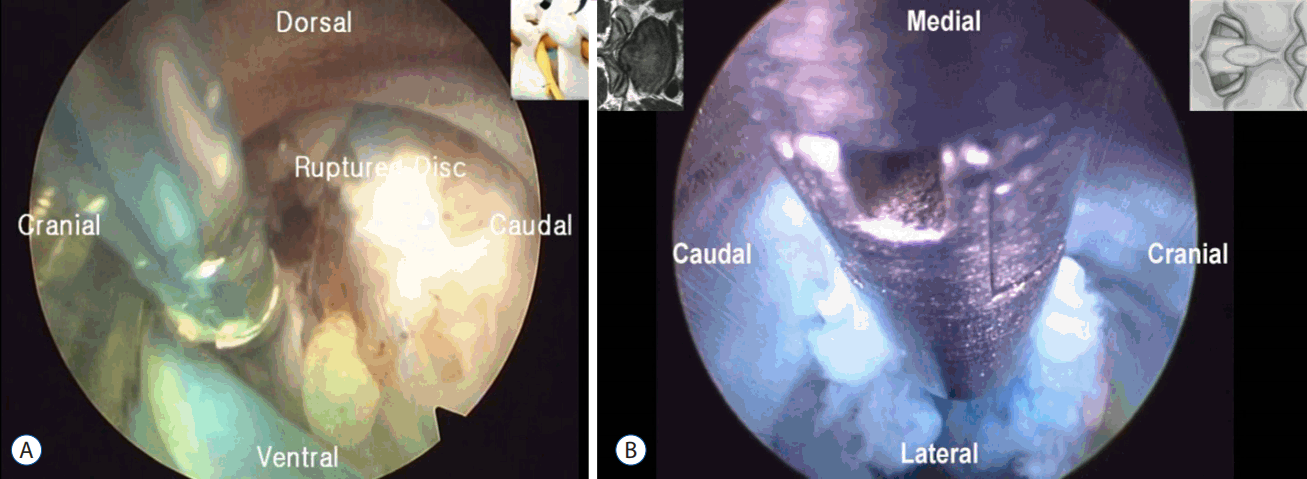
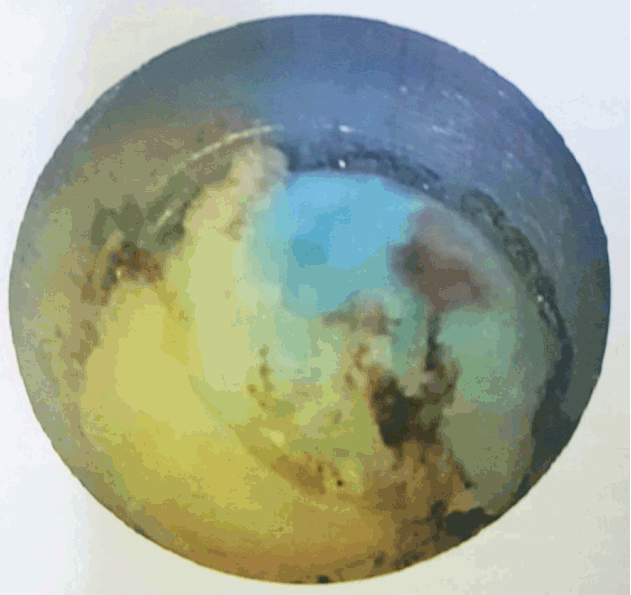
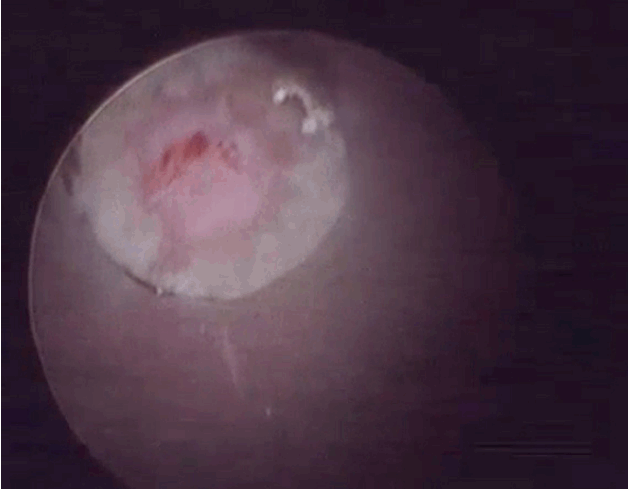
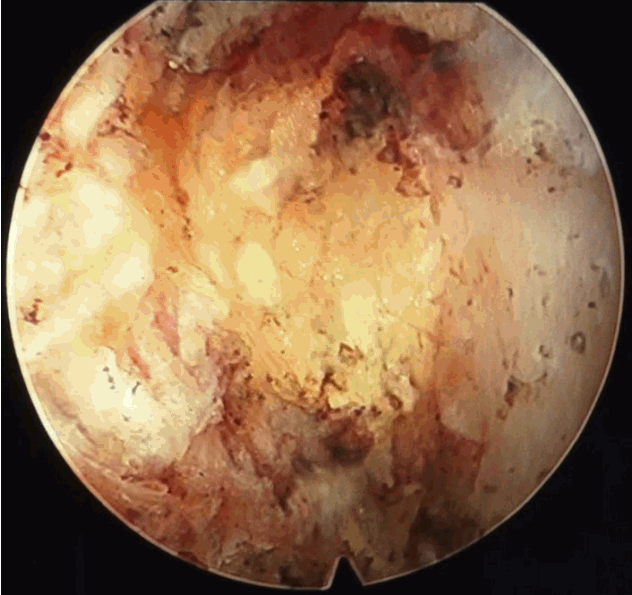
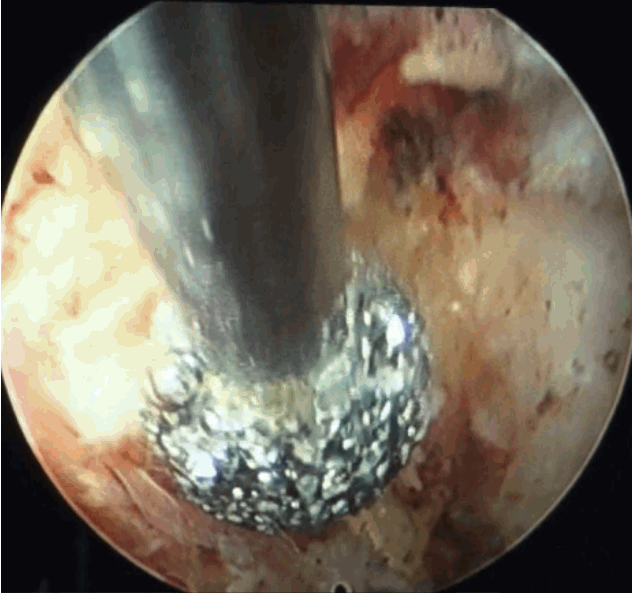
- After introduction of endoscope try to identify the structures like epidural fat, annular tear with nuclear caught fragment, posterior longitudinal ligament, traversing nerve root, exiting nerve root, superior facet and superior and inferior pedicular notches (Fig. 5). Remove the fragment with different types of forceps and clean the annular tear with bipolar cold coagulation (Elliquence, Baldwin, NY, USA) and Ho-YAG laser if needed.
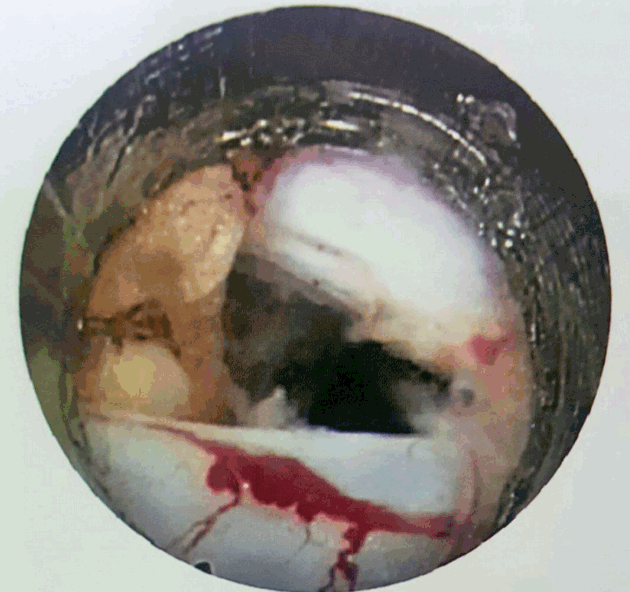
- Free movement of thecal sac and traversing root, fresh epidural bleeding, and subsidence of pain are the signs of an adequate decompression.
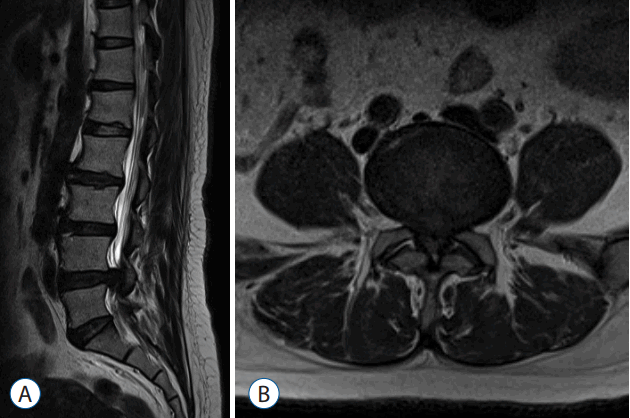
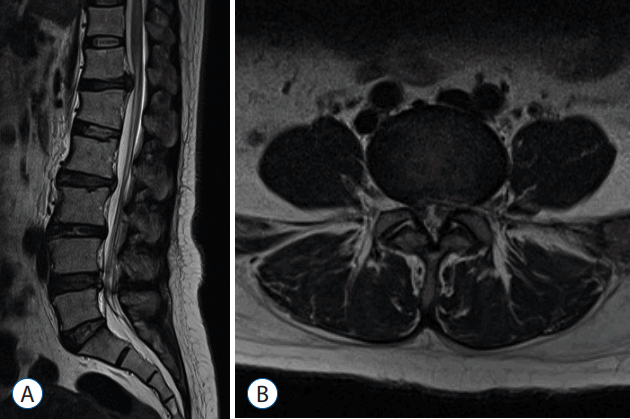
- Pre-operative (Fig. 6) and post-operative MRI (Fig. 7) shows there is complete removal of herniated fragment.
Interlaminar approach
Surgical steps
- Once location of disc herniation is determined on images needle entry point is to be calculated from images. Entry point should be in reference to the medial pedicular line in AP and spino-laminar line on lateral view with direction of needle towards superior endplate of S1 vertebra (Fig. 8). For shoulder herniation needle to be directed at superomedial aspect of pedicle and axillary herniation it should be directed towards lower lamina (midpoint of pedicle and spinous process).
- Once needle reaches to spinolaminar junction, the needle tract is dilated with series of dilators and endoscope (Spine Doctors) to be introduced. First structure encountered is ligamentum flavum which when splitted, epidural fat is seen (Fig. 9). Bipolar radiofrequency coagulation is used to remove fat and next structure which is seen is neural tissue. Working cannula is used to guard S1 root and herniation to be identified and removed with forceps (Fig. 10). Confirm a free movement of the nerve root (Fig. 11).
Complications
- Immediate complications: 1) injury to the neural and vascular structures, 2) perforation of peritoneal sac and abdominal contents, 3) missed fragments, 4) exploration of wrong level or wrong side, and 5) instrument breakage
- Early complications: 1) psoas hematoma, 2) postoperative hematoma formation, 3) cerebrospinal fluid cyst formation, and 4) infection
- Delayed complications: 1) recurrent disc herniation and 2) possible instability
ENDOSCOPIC CERVICAL SPINE SURGERY
Percutaneous endoscopic cervical discectomy (PECD)
Surgical approach
- Patient is placed supine on radiolucent operating table with neck slightly extended by placing pillow between two shoulders and head is stabilised with adhesive tape, both shoulders are pulled down with adhesive tape to visualise lower cervical spine under C-arm. Level is marked under C-arm and operative field prepared and draped (Fig. 12).
- Approach is usually opposite site of the herniation for paracentral and foraminal disc herniation and for central disc herniation right side for right handed surgeon and vice versa. Infiltrate the skin with 1% lidocaine along with conscious sedation given preoperatively (Intravenous Midazolam 0.05 mg/kg and 0.8 mg/kg Fentanyl and can be repeated if required).
- Palpate the carotid pulse with left hand. Trachea-oesophageal complex is pushed away by finger nails and feel anterior vertebral surface. So the safe interval is created by fingers of left hand and confirmed under C-arm.
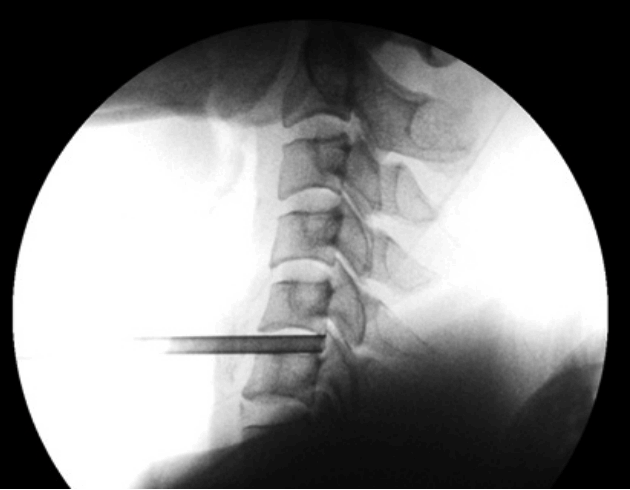
- Under fluoroscopy guidance insert 18 gauge needle through skin from the safe interval and advance till anterior disc surface, insert a needle from the centre of the disc between two longus colli to prevent injury to the cervical sympathetic chain and bleeding (Fig. 13).
- Perform a discography with 0.5 mL admixture of radio opaque dye, normal saline and indigo carmine in the ratio of 2: 2: 1, it help to identify herniated fragment as its stains acidic degenerated nucleus pulposus to blue (Fig. 14).
- Exchange needle with guide wire, make skin incision of around 5 mm and introduce series of dilators. Finally replace it with appropriate size working cannula.
- Tip of cannula should be at posterior vertebral line in lateral view and according to the location of herniation in AP C-arm view (Fig. 15).
- Introduce the cervical endoscope (Spine Doctors) and remove herniated disc fragments (Fig. 16). If fragments are caught inside annulus use side firing Ho: YAG laser to make circular annular window to access the fragment (Fig. 17).
- Pulsating dura and posterior annulus is a sign of complete removal of fragments. Remove endoscope and cannula, close incision with single stitch (Fig. 18).
Percutaneous endoscopic posterior cervical foraminotomy
Surgical steps
- Under general anaesthesia, patient is placed in prone and reverse trendelenburg’s position (Fig. 19)
- Under fluoroscopy, facet joint is identified and needle is introduced (Fig. 20), followed by guide wire and sequential dilators (Fig. 21) and finally replaced with working cannula (6.5 mm) (Fig. 22). Introduce the endoscope (Spine Doctors) and visualise facet junction (‘V’ shaped area) (Fig. 23).
- Further procedure requires drilling of facet with burr and kerrison punches but there should be less than 50% of facet joint drilling (Figs. 24–26). Visualise the axilla and remove herniated fragment.
- Close the wound with single stitch.
Complications of cervical endoscopy
- Neurological injury like damage to the cervical cord or nerve root due to inadvertent use of forceps or laser (transient with laser).
- Vascular injury like carotid vessels during PECD and vertebral artery while foraminotomy.
- Visceral injury mainly oesophagus because it is soft collapsible tube and highly prone to injury while needle insertion in PECD.
Percutaneous endoscopic thoracic discectomy
Indications
- Soft thoracic disc herniation
- Axial back pain and/or radicular pain including the following
- Interscapular pain
- Thoracolumbar pain
- Anterior radiating chest pain
- Intercostal pain or low back pain
- Mild degree of myelopathy due to soft disc herniation without calcification
- Failure of adequate conservative therapy




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download