Abstract
Objective
The aim of this study to investigate the normal values of erythropoietin (EPO) and neuroprotective effects of quercetin and mannitol on EPO and hematocrit levels after acute severe traumatic brain injury (TBI) in rat model.
Methods
A weight-drop impact acceleration model of TBI was used on 40 male Wistar rats. The animals were divided into sham (group I), TBI (group II), TBI+quercetin (50 mg/kg intravenously) (group III), and TBI+mannitol (1 mg/kg intravenously) (group IV) groups. The malondialdehyde, glutathione peroxidase, catalase, EPO, and hematocrit levels were measured 1 and 4 hour after injury. Two-way repeated measures analysis of variance and Tukey’s test were used for statistical analysis.
Results
The malondialdehyde levels decreased significantly after administration of quercetin and mannitol compared with those in group II. Catalase and glutathione peroxidase levels increased significantly in groups III and IV. Serum EPO levels decreased significantly after mannitol but not after quercetin administration. Serum hematocrit levels did not change significantly after quercetin and mannitol administration 1 hour after trauma. However, mannitol administration decreased serum hematocrit levels significantly after 4 hour.
Traumatic brain injury (TBI) is a common cause of death and disability in young people and a major health and socioeconomic problem worldwide9,18,32,46). TBI can result in permanent cognitive and motor deficits due to primary and secondary injuries10). Many serum biomarkers have been used to detect the severity of TBI. Glial protein S-100 beta (S100B), glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 (UCH-L1) serum levels incerases according to the severity of the TBI31). Neurological damage occurs mostly from secondary brain injury caused by brain edema, increased intracranial pressure, and decreased cerebral perfusion11). However, no effective treatments are available for the secondary injury except routine medical intervention and care36,38).
Quercetin is a well-known flavonoid found widely in red onions, grapes, apples, berries, cherries, broccoli, citrus, lettuce, chili peppers, tomatoes, apple nuts, tea, potatoes, soybeans, peanuts, and red wine5,22,24). Quercetin can cross the blood–brain barrier (BBB) and has neuroprotective effects on the central nervous system27,40,50). Quercetin also has cardioprotective, antioxidant, anti-inflammatory, anti-apoptotic, anti-tumoral, anti-thrombotic, antiviral, and antidepressant properties5,15,16,21,26,34,44).
Quercetin reduces oxidative stress and lipid peroxidation by increasing catalase (Cat) and glutathione peroxidase (GSH-Px) levels, which have an important roles as antioxidants2,30). Malondialdehyde (MDA) is the endproduct of lipid peroxidation and is a commonly used marker of oxidative stress42).
Mannitol is a hypertonic, hyperosmolar agent commonly used to treat vasogenic, cytotoxic, and interstitial brain edema29). Mannitol also has an antioxidant effect by reducing oxygen free radicals49).
Erythropoietin (EPO) and the EPO receptor (EPOR) play important roles in erythropoiesis and are generally detected in the kidney and liver17). Tissue hypoxia activates EPO, which has antioxidative, anti-inflammatory, anti-apoptotic, neurotrophic, and angiogenic effects47). EPO and EPOR are also expressed in the central nervous system, where they have a neuroprotective effect after TBI1).
Low hematocrit level increases intracranial pressure after traumatic brain injury51). Hematocrit levels should not be lower than 30% in clinical practice45).
In this study, we investigated the normal EPO values and the neuroprotective effects of quercetin and mannitol on EPO and hematocrit levels after acute severe TBI in a rat model.
All experimental procedures and animal care were in accordance with the guidelines of Osmangazi University, Faculty of Medicine. The experimental protocols used in this study were approved by the Animal Experimental Ethics Committee of Dokuz Eylul University.
A weight-drop impact acceleration model of TBI was used on the rats in this study6,33). Forty male Wistar rats (weight, 318.3±5 g) were divided randomly into four groups (n=10/group): group I, sham; group II, TBI; group III, TBI+quercetin (50 mg/kg intravenous); and group IV, TBI+mannitol (1 mg/kg intravenous). The rats were anesthetized intraperitoneally with 100 mg/kg s-ketamine (Ketanest S; Pfizer, New York, NY, USA) and 10 mg/kg xylazine (Xylazin 2%; Medistar, Ascheberg, Germany)7).
The rats were acclimatized in a suitable environment (21±2°C and 60±5% humidity) for 1 week before starting the experiment. The body weight of each rat was determined before surgery. A heating pad and heating lamp were used to maintain a rectal temperature of 36.5–37.5°C. Rats with a skull fracture, seizure, or nasal bleeding or that did not survive the impact were excluded from the study. All rats were sacrificed at 4 h after TBI.
Blood (50 μL) was collected via a tail vein before injury and 1 and 4 h after TBI to determine the MDA (R&D Systems, Minneapolis, MN, USA), Cat (Cusabio Biotec ELISA kit, Wuhan, China), GSH-Px (Cusabio Biotec), and EPO (Sigma Aldrich Chemie GmbH, Steinheim, Germany) levels in serum using enzyme-linked immunoassay kits according to the manufacturer’s instructions.
Statistical analyses were performed with the SigmaStat 3.5 package (Systat Software Inc., San Jose, CA, USA). Data are presented as mean±standard deviation. A two-way repeated-measures analysis of variance (ANOVA) was used to evaluate interactions among the groups, and Tukey’s test was used to detect differences between groups and the repeated measures. A p-value <0.05 was considered significant.
The MDA levels in groups III and IV were significantly lower than those in group II (p<0.005). The Cat levels were significantly higher in groups III and IV than those in group II (p<0.001) (Table 1). However, no difference in Cat levels was observed between groups III and IV (p=0.236). The GSH-Px levels were significantly higher in groups III and group IV than those in group II after TBI (p<0.005) (Table 2). Quercetin more effective on GPH-Px levels compared with that of mannitol but there was no significant difference (p<0.152) (Fig. 1).
The serum EPO level in group II increased compared with that in group I. No differences were detected between the Cat (p=0.576) and GSH-Px (p=1.415) levels 1 and 4 h after TBI. Quercetin tended to decrease the EPO levels (p=0.102), whereas mannitol significantly decreased the serum EPO levels (p<0.005). Our data revealed that administering quercetin after TBI did not negatively change the EPO levels, whereas mannitol lowered them significantly. Serum hematocrit did not change significantly 1 h after TBI in response to quercetin (p=0.170) or mannitol (p=0.130) administration. However, mannitol decreased serum hematocrit significantly 4 h after TBI (p<0.05) because mannitol has an osmotic effect on blood plasma (Fig. 2). These results indicate that mannitol, but not quercetin, changed the serum EPO levels after TBI.
TBI is a pathological condition with debilitating and possibly lethal features as a result of primary and secondary damage. Brain swelling has an important role in secondary brain injury, as it results in increased intracranial pressure and decreased cerebral perfusion leading to neuronal death11). Several drugs, such as antioxidants and calcium channel blockers, have been developed to prevent the secondary injury caused by TBI; however, none are clinically effective.
A weakness of our study is that we studied biochemical parameters in blood. Some authors studied biochemical parameters in traumatic brain tissue44,49). Hematocrit normally increases within 2 weeks and returns to normal within 6 weeks after trauma13,35). Hematocrit changes drastically after a massive blood loss, dehydration, or hyper-hydration in patients suffering from acute traumatic injury.
The proposed neuroprotective effect of quercetin is that it directly decreases oxidative stress and iron-mediated lipid peroxidation and inhibits myeloperoxidase activity and the apoptotic pathways12). Few studies have reported the levels of GSH-Px, Cat, and MDA after administration of quercetin and mannitol in cases of TBI.
Quercetin has a neuroprotective effect by increasing GSH-Px and decreasing the MDA levels after subarachnoid hemorrhage in rats. In our study, quercetin increased the GSH-Px and Cat levels but significantly decreased the MDA level.
Schültke et al. reported that quercetin prevented the decrease in glutathione and MDA after fluid percussion injury in a rat model44). In the present study, quercetin was more effective than mannitol on MDA and GSH-Px levels in the rat drop-weight model. Ossola et al. reported that quercetin did not cross the BBB; however, other studies have demonstrated that it does cross the BBB14,37). Mannitol can cross and disrupt the BBB, which helps other drugs to cross the BBB. However, the BBB is often damaged after TBI, which can allow other drugs to cross the BBB as well. Low-dose mannitol provides improved the efficiency of quercetin in our study and could be used in a combined treatment with quercetin in future studies. Quercetin may increase antioxidant enzyme activities (GSH-Px and Cat) and improve cognitive function after TBI48). Our study demonstrated that quercetin activated antioxidant enzymes and reduced lipid peroxidation.
Mannitol and hypertonic saline increase Cat and GSH-Px levels; thus reducing the MDA level49). In the present study, mannitol lowered MDA and increased GSH-Px and Cat levels immediately after TBI, but quercetin had more of an effect on MDA and GSH-Px levels than did mannitol.
Preclinical studies have shown that EPO protects against neurological injury in several in vitro and in vivo experimental models19,20,28,41). In our study, the EPO levels increased after trauma, presumably to promote neuroprotection, but quercetin and mannitol decreased the EPO levels. Further, no differences in the EPO levels were observed between the groups after quercetin or mannitol administration. We demonstrated that mannitol reduced the EPO levels after TBI. Basarslan et al. found that EPO reduced tissue MDA levels and increased GSH-Px activity in a group that received EPO. They concluded that saline and dextran had no effect on lipid peroxidation in an experimental rat drop-weight model4). Quercetin and mannitol also reduced the MDA level and increased GSH-Px activity for neuroprotection, similar to EPO. Administering EPO and maintaining hemoglobin >10 g/dL in patients with TBI did not result in neurobehavioral improvement, but neurological improvement occurred after 6 months41). Peng et al. reported that EPO helped to treat experimental TBI by reducing lesion volume and improving neurological outcome39).
Administering EPO to rats with traumatic axonal injury increased the expression of EPOR, which plays an important role in neuroprotection25). Schober et al. reported that EPO inhibited caspase-dependent apoptosis and improved neurobehavioral outcomes early after a controlled cortical impact43).
One study administered EPO 30 min after diffuse impact-acceleration and evaluated the animal 2 h later8). They found that brain hypoxia and cell edema were reversed by recombinant human EPO. Hartley et al. demonstrated that EPO increased extracellular glucose levels and decreased lactate and pyruvate levels after acute TBI in a rat model, which maintained the energy requirements of the brain23). In our study, we showed a neuroprotective effect of EPO in an acute severe rat TBI model.
EPO significantly increases hematocrit in the long term after TBI. Zhang et al. reported that an increased hematocrit level did not affect the neuroprotective or neurorestorative effects of EPO in rats after TBI, suggesting that the effect of EPO was independent of hematocrit51). Balak et al. demonstrated that serum osmolarity decreased during the first 3 h after TBI3). We failed to show a significant role of hematocrit as an independent parameter, and quercetin had no effect on hematocrit. Hematocrit levels did not change 1 h after TBI, but decreased after 4 h in group IV, possibly because of the osmotic effect of mannitol on blood plasma.
References
1. Akdemir Ozisik P, Oruckaptan H, Ozdemir Geyik P, Misirlioglu M, Sargon MF, Kilinc K, et al. Effect of erythropoietin on brain tissue after experimental head trauma in rats. Surg Neurol. 68:547–555. discussion 555. 2007.

2. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 20:269–275. 2009.

3. Balak N, Isiksacan N, Turkoglu R. Does serum osmolarity change as a result of the reflex neuroprotective mechanism of cerebral osmo-regulation after minor head trauma? J Korean Neurosurg Soc. 45:151–156. 2009.

4. Basarslan SK, Gocmez C, Kamasak K, Ekici MA, Ulutabanca H, Dogu Y, et al. The effects of erythropoietin, dextran and saline on brain edema and lipid peroxidation in experimental head trauma. Ulus Travma Acil Cerrahi Derg. 21:235–240. 2015.
5. Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 11:733–740. 2008.

6. Blaha M, Schwab J, Vajnerova O, Bednar M, Vajner L, Michal T. Intracranial pressure and experimental model of diffuse brain injury in rats. J Korean Neurosurg Soc. 47:7–10. 2010.

7. Bleilevens C, Roehl AB, Goetzenich A, Zoremba N, Kipp M, Dang J, et al. Effect of anesthesia and cerebral blood flow on neuronal injury in a rat middle cerebral artery occlusion (MCAO) model. Exp Brain Res. 224:155–164. 2013.

8. Bouzat P, Millet A, Boue Y, Pernet-Gallay K, Trouve-Buisson T, Gaide-Chevronnay L, et al. Changes in brain tissue oxygenation after treatment of diffuse traumatic brain injury by erythropoietin. Crit Care Med. 41:1316–1324. 2013.

9. Cole TB. Global road safety crisis remedy sought: 1.2 million killed, 50 million injured annually. JAMA. 291:2531–2532. 2004.
10. Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit Care Nurs Q. 23:1–13. 2000.

11. DeWitt DS, Jenkins LW, Prough DS. Enhanced vulnerability to secondary ischemic insults after experimental traumatic brain injury. New Horiz. 3:376–383. 1995.
12. Dong YS, Wang JL, Feng DY, Qin HZ, Wen H, Yin ZM, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 11:282–290. 2014.

13. Dugas AJ Jr, Castaneda-Acosta J, Bonin GC, Price KL, Fischer NH, Winston GW. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J Nat Prod. 63:327–331. 2000.

14. Faria A, Pestana D, Teixeira D, Azevedo J, De Freitas V, Mateus N, et al. Flavonoid transport across RBE4 cells: a blood-brain barrier model. Cell Mol Biol Lett. 15:234–241. 2010.

15. Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol. 539:168–176. 2006.

16. Ferrali M, Signorini C, Ciccoli L, Bambagioni S, Rossi V, Pompella A, et al. Protection of erythrocytes against oxidative damage and autologous immunoglobulin G (IgG) binding by iron chelator fluor-benzoil-pyridoxal hydrazone. Biochem Pharmacol. 59:1365–1373. 2000.

17. Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood). 228:1–14. 2003.

19. Grasso G, Alafaci C, Buemi M. Erythropoietin in traumatic brain injury: an answer will come soon. World Neurosurg. 84:1491–1492. 2015.

20. Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 1182:99–105. 2007.

21. Haleagrahara N, Radhakrishnan A, Lee N, Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol. 621:46–52. 2009.

22. Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 54:9966–9977. 2006.

23. Hartley CE, Varma M, Fischer JP, Riccardi R, Strauss JA, Shah S, et al. Neuroprotective effects of erythropoietin on acute metabolic and pathological changes in experimentally induced neurotrauma. J Neurosurg. 109:708–714. 2008.

24. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.

25. Hellewell SC, Yan EB, Alwis DS, Bye N, Morganti-Kossmann MC. Erythropoietin improves motor and cognitive deficit, axonal pathology, and neuroinflammation in a combined model of diffuse traumatic brain injury and hypoxia, in association with upregulation of the erythropoietin receptor. J Neuroinflammation. 10:156. 2013.

26. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 342:1007–1011. 1993.

28. Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 65:485–492. 2009.

29. Kaufmann AM, Cardoso ER. Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J Neurosurg. 77:584–589. 1992.

30. Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 49:1712–1720. 2008.

31. Lee JY, Lee CY, Kim HR, Lee CH, Kim HW, Kim JH. A role of serum-based neuronal and glial markers as potential predictors for distinguishing severity and related outcomes in traumatic brain injury. J Korean Neurosurg Soc. 58:93–100. 2015.

32. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7:728–741. 2008.

33. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 80:291–300. 1994.
34. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 52:673–751. 2000.
35. Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 32:386–393. 2002.

36. Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 19:503–557. 2002.

37. Ossola B, Kaariainen TM, Mannisto PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 8:397–409. 2009.

38. Park JE, Kim SH, Yoon SH, Cho KG, Kim SH. Risk factors predicting unfavorable neurological outcome during the early period after traumatic brain injury. J Korean Neurosurg Soc. 45:90–95. 2009.

39. Peng W, Xing Z, Yang J, Wang Y, Wang W, Huang W. The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models. J Neurosurg. 121:653–664. 2014.

40. Rangel-Ordonez L, Noldner M, Schubert-Zsilavecz M, Wurglics M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761®. Planta Med. 76:1683–1690. 2010.

41. Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 312:36–47. 2014.

42. Rossi R, Dalle-Donne I, Milzani A, Giustarini D. Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem. 52:1406–1414. 2006.

43. Schober ME, Requena DF, Block B, Davis LJ, Rodesch C, Casper TC, et al. Erythropoietin improved cognitive function and decreased hippocampal caspase activity in rat pups after traumatic brain injury. J Neurotrauma. 31:358–369. 2014.

44. Schültke E, Kamencic H, Zhao M, Tian GF, Baker AJ, Griebel RW, et al. Neuroprotection following fluid percussion brain trauma: a pilot study using quercetin. J Neurotrauma. 22:1475–1484. 2005.

45. Tango HK, Schmidt AP, Mizumoto N, Lacava M, Cruz RJ Jr, Auler JO Jr. Low hematocrit levels increase intracranial pressure in an animal model of cryogenic brain injury. J Trauma. 66:720–726. 2009.

46. Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 282:954–957. 1999.

47. Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: a double-edged sword? Pharmacol Ther. 128:445–459. 2010.

48. Yang T, Kong B, Gu JW, Kuang YQ, Cheng L, Yang WT, et al. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell Mol Neurobiol. 34:797–804. 2014.

49. Yilmaz N, Dulger H, Kiymaz N, Yilmaz C, Gudu BO, Demir I. Activity of mannitol and hypertonic saline therapy on the oxidant and antioxidant system during the acute term after traumatic brain injury in the rats. Brain Res. 1164:132–135. 2007.

Fig. 1
A: Serum malondialdehyde (MDA) levels (mean±standard error [SEM]*) in the groups 1 and 4 h after traumatic brain injury (TBI). The MDA levels decreased significantly in groups III and IV compared with that in group II after TBI (p<0.005). B: The serum Catalase (Cat) levels (mean±SEM*) in the groups 1 and 4 h after TBI. The Cat levels increased significantly in groups III and IV compared with those in group II after TBI (p<0.001). C: The serum Glutathione peroxidase (GSH-Px) levels (mean±SEM*) in the groups 1 and 4 h after TBI. The GSH-Px levels increased significantly in groups III and IV compared with those in group II after TBI (p<0.005). *MDA levels were significantly lower than group II (p<0.05). †Catalase levels were significantly higher than group II (p<0.05). ‡GSH-Px levels were significantly higher than group II (p<0.05).
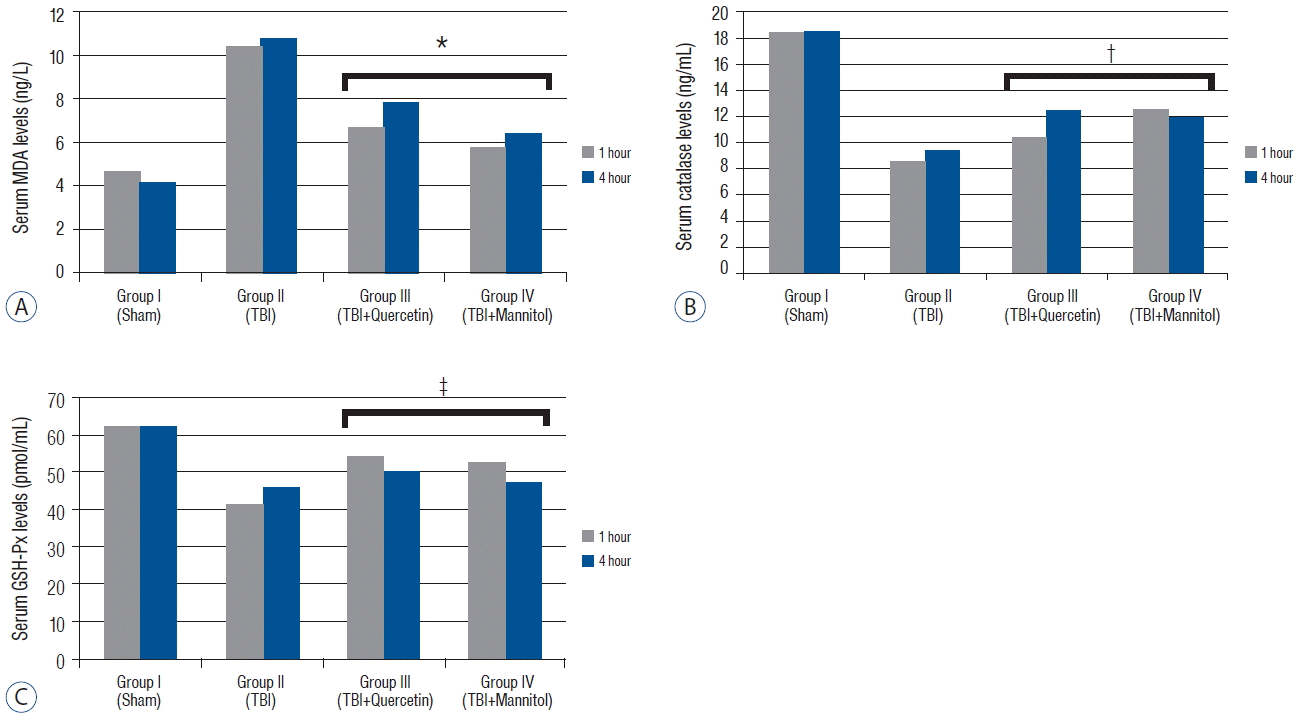
Fig. 2
A: Serum erythropoietin (EPO) levels (means±standard error [SEM]*) in the groups 1 and 4 h after traumatic brain injury (TBI). The serum EPO level tended to decrease in group III compared with that in group I after TBI (p=0.102). The serum EPO levels decreased significantly in group IV compared with that in group I after TBI (p<0.005). B: The hematocrit (HTC) levels (mean±SEM*) in the groups 1 and 4 h after TBI. The serum HTC levels decreased significantly in group IV 4 h after TBI (p<0.05). *EPO levels were significantly lower than group II (p<0.05). †HTC levels are were significantly lower than group II (p<0.05).
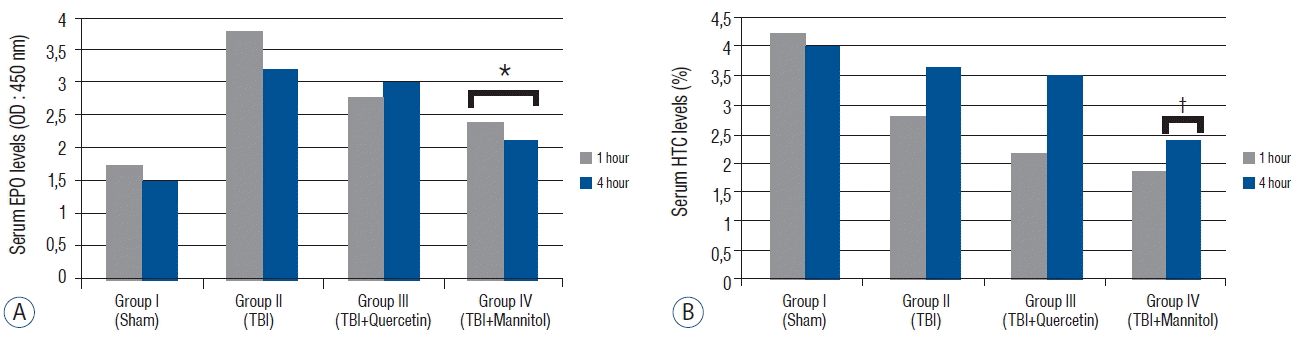
Table 1
Statistical analysis of 1 hour after TBI
| 1 hour | Sham (n=10) | Trauma (n=10) | Quercetin (n=10) | Mannitol (n=10) |
|---|---|---|---|---|
| MDA (ng/L) | 4.71±0.41 | 10.38±1.15 | 6.71±0.44* | 5.84±0.78* |
| Catalase (ng/mL) | 18.38±2.70 | 8.63±1.73 | 10.40±1.62† | 12.50±2.76† |
| GSH-Px (pmol/mL) | 61.90±6.65 | 41.10±2.79 | 54.00±6.60‡ | 52.70±12.44‡ |
| EPO (OD: 450 nm) | 1.70±0.34 | 3.80±0.44 | 2.80±0.72 | 2.40±0.66§ |
| HTC (%) | 4.21±0.41 | 2.80±0.82 | 2.17±0.17 | 1.85±0.31 |
Table 2
Statistical analysis of 4 hour after TBI
| 4 hour | Sham (n=10) | Trauma (n=10) | Quercetin (n=10) | Mannitol (n=10) |
|---|---|---|---|---|
| MDA (ng/L) | 4.23±0.81 | 10.75±1.57 | 7.80±1.52* | 6.43±1.95* |
| Catalase (ng/mL) | 18.55±1.56 | 9.40±0.66 | 12.40±0.96† | 11.90±2.55† |
| GSH-Px (pmol/mL) | 62.20±6.13 | 45.60±6.21 | 49.90±7.37‡ | 47.20±6.91‡ |
| EPO (OD: 450 nm) | 1.50±0.44 | 3.20±0.54 | 3.00±0.50 | 2.10±0.40§ |
| HTC (%) | 4.01±1.29 | 3.65±0.94 | 3.50±0.93 | 2.40±0.30|| |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download