INTRODUCTION
Hydrocephalus is a spectrum of conditions where there is a mismatch of cerebrospinal fluid (CSF) production and absorption, with resultant enlarged ventricles. There are many proposed classifications for hydrocephalus. Most commonly in use is the obstructive (non-communicating) and the communicating type7). In the obstructive variety, the block is proximal to the arachnoid granulations and may be further classified into intraventricular or extraventricular41). The communicating type develops because of defective absorption at the arachnoid granulation. Other classifications include etiologic classification viz. tumor related, post-hemorrhagic, post-infectious, post-traumatic, ex vacuo and normal pressure hydrocephalus.
Over a hundred years ago, pioneers like Dandy, Lespinasse, and Mixter attempted to treat hydrocephalus with open and endoscopic third ventriculostomy (ETV), choroid plexectomy and fulguration. Their poor results prompted the medical and neurosurgical community to look for other solutions. This came in the form of shunts devised by Nulsen and Spitz working with an engineer Holter in the 1950’s44). This technology was immediately accepted and has further evolved and matured to become the standard of care for all types of hydrocephalus. However, in spite of several innovations and technical modifications, shunts are not without complications and have remained a constant source of concern for the child, parents and the family. Along with improvements in endoscope and camera technologies, desire of shunt freedom has led to a resurgence of ETV techniques.
ETV is recommended as the choice of treatment for obstructive hydrocephalus in many centers now. Though the immediate success of this procedure can be determined by clinical improvement and absence of need for a further surgical procedure, there are several concerns in assessing the outcome. The influence of age, etiology and previous shunting has been frequently reported and predictive scores and guidelines have been described. However, major worrisome factors for long-term outcome are the suboptimal reduction in ventricular size and resultant failure of restoration of cortical mantle as well as persistent large head. Furthermore, the place of repeat ETV and other endoscopic solutions in complex hydrocephalus are being evaluated. We have tried to review these factors in available literature with comments from our experience.
DISCUSSION
Success of ETV
The simplest definition of success of ETV is “freedom from a shunt”.
Evolution of ETV for hydrocephalus has been a lesson in history of how a neurosurgical procedure can and should be evaluated with technical innovations and better case selection as described by Schmitt and Jane44).
Patient parent support groups like the Hydrocephalus Association in the USA have defined the success of the procedure thus15) - “Success in terms of this procedure is usually considered (by patients and doctors alike) to be avoiding a shunt in a patient who would otherwise require one. Most doctors would categorize ETV as successful if a patient later shows clinical evidence of normal intracranial pressure (ICP) and structural evidence of stable or decreased ventricular size. If a patient was previously shunted, the shunt must be either removed or proved nonfunctional to demonstrate success.”
DETERMINANTS OF SUCCESS
Several factors contribute to the success of the ETV.
Clinical selection for ideal candidate
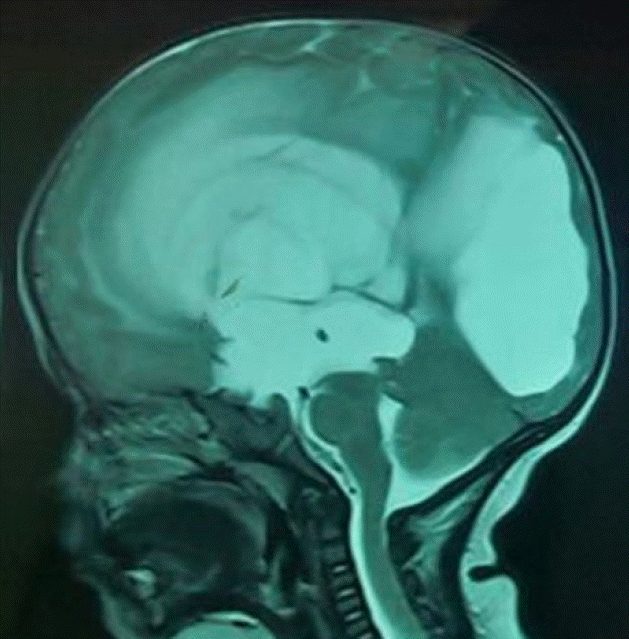
Age greater than 6 months with aqueduct stenosis or tectal tumor and no previous shunting can be considered as an ideal candidate for ETV. ETV works best as a primary procedure in obstructive hydrocephalus without evidence of prior infection or hemorrhage (Fig. 1). Patients with communicating hydrocephalus are usually not good candidates for this technique although some groups claim success20,40).
Radiologically ideal ETV candidate
Presence of clear evidence of ventricular non-communication i.e. obstructive pattern of hydrocephalus, anatomic obstruction at the aqueduct and/or lack of aqueduct flow void on T2 magnetic resonance imaging (MRI) and a thinned out floor of the third ventricle with a downward bulge are good indications for ETV. Width of foramen of Monroe should be sufficient to accommodate the endoscope (7 mm for rigid scope, 4 mm for flexible scope).
Technical considerations: Intraoperative criteria for ideal ETV candidate
The perforation of the floor should preferably be made without application of an energy source. Openings made using a monopolar energy source are believed to induce an inflammatory response which may close the stoma. Some groups have used a contact laser to create the opening. A high power Nd-YAG or lower powered diode lasers have been used8). These groups have shown favorable results. Our policy is to use the blunt end of a monopolar probe without application of any energy, to make the stoma. The stoma size should be adequate, usually around 3–4 mm in diameter. A rough estimate of adequacy is the ability to insert the scope through the stoma into the pre-pontine cistern. The stoma size is enlarged with a 3 or 4 Fr Fogarty balloon catheter. This is an important step during the procedure. An additional advantage is that minor capillary bleeding can be halted by inflating the balloon to tamponade the bleeding vessels. Visualization of perforating branches of the basilar artery along with opening up of any second membrane is routinely considered satisfactory enough.
A study of 403 patients by Warf and Kulkarni54) observed that scarring at the cisterns more than doubled the risk of ETV failure and an open aqueduct increased the risk of failure by 50%. The success rate has been shown to be directly proportional to the experience of operating surgeon and volume of cases tackled by the pediatric centre apart from the above mentioned factors13). Usually at the end of the procedure, with the irrigation turned off, the floor of the third ventricle can be seen pulsating or flapping with arterial pulsations and the respiratory movements.
In all cases of secondary ETV or in cases there has been some bleeding during the procedure, an Ommaya reservoir and ventricular catheter are inserted for access. This is helpful in acute hydrocephalus due to blockage of the stoma.
CRITERIA FOR DEFINING SUCCESS
Clinical
This includes resolution of the preoperative signs of raised ICP i.e., improvement in the level of consciousness, resolution of eye movement abnormalities, resolution of headaches, stabilization or reduction in the head circumference and reduction of the fontanelle tension (in infants).
Radiological
On appropriate temporal follow up, criteria include decrease or stabilization of the ventricular size over a period of three months. Most marked change is noted in cases of acute hydrocephalus and in reduction in size of the third ventricle (i.e., by 25%)42). A 15% reduction in size of the third ventricle within 1 month is considered as a reliable indicator of favorable outcome47). Usually the extent of ventricular size reduction postoperatively is inversely proportional to the duration and magnitude of symptoms preoperatively46). It has been noted that sometimes the ventricular size may not show a remarkable reduction on imaging post ETV as compared to post shunting. However, this need not be a reflection of raised ICP. Conversely reduction in ventricular size post ETV may or may not indicate a successful procedure as it has been found to reduce marginally in both settings. A greater reduction in mean ventricular size (16% vs. 7 %) was observed by Kulkarni et al.27) in successful ETV outcomes as compared to ETV failures. Twenty nine children who underwent ETV were followed up here. Flow voids were present in 94% of successful cases and absent in 75% of failures. Evans ratio, third ventricle index, cella media index (ratio of the biparietal diameter of skull to maximum external diameter of lateral ventricles at the central part of lateral ventricles) and ventricular score has been found to decrease in favorable outcome cases and increase in failed ones43).
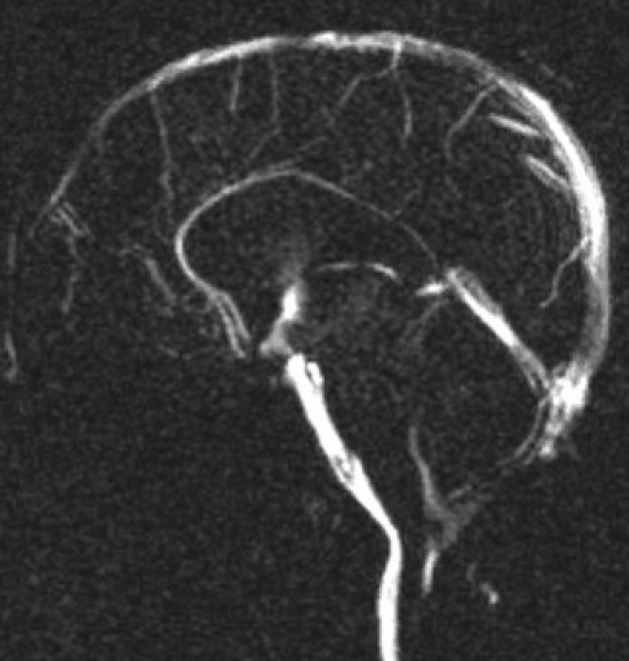
Special MRI sequences like the phase contrast cine flow study (Fig. 2), heavily weighted T2 studies and more recently 3D-SPACE sequences are being used to assess patency of the stoma1). Sometimes, a computed tomography (CT) contrast dye study may be used if one has ventricular access through an Ommaya reservoir or a similar device. Here radiopaque contrast is injected into the ventricles via the reservoir and sequential CT scans in time reveal the migration of the dye from the lateral ventricle to the brainstem cisterns and absorption in case of a patent stoma.
Thus clinical correlation is more important than radiological correlation to determine successful outcome. Imaging does provide a reliable mean to positively predict the patients’ course but its use as a sole entity is inadequate5).
COMPLICATIONS AND ITS AVOIDANCE
Complications include bleeding from the walls of the third ventricle. This is usually temporary and stops with irrigation and sometimes balloon tamponade. Larger vessel bleeding like perforators or basilar artery branches can lead to significant morbidity. Basilar artery aneurysms/pseudo-aneurysms may result from this hemorrhage. Hypothalamic injury may result from eccentric perforations. This again has a very high morbidity. Perforation of the floor in ETV can theoretically affect the hypothalamo-pituitary axis leading to hormonal disturbances. Fritsch et al. examined 20 such patients but found no clinical relevance as such. Further studies are needed to ascertain the endocrine outcome post ETV18). Cranial nerve injury can result from improper placement of the balloon catheter and inflation causing stretch of the nerves. Cardiac arrest is a possibility in case of over inflation of the balloon accompanied by failure of egress of the irrigating fluid. CSF leak and electrolyte imbalance can be challenging complications to treat. Post-operative CSF leak from the wound is an indicator of poor outcome. Subdural effusions are also known to occur. Occlusion of the site of ETV leading to sudden death is a rare occurrence but has been known to occur11). Closure of the stoma secondary to gliosis usually presents within 2 years unlike acute shunt malfunction which may manifest till many years post shunt insertion necessitating the need for a longer follow-up. An Ommaya inserted in select high risk cases may turn out to be life-saving by helping to achieve a rapid ventricular access to negate the non-patent stoma32).
ETV SUCCESS SCORE AS A PREDICTIVE TOOL
The most commonly used tool to predict the chances of a successful ETV are the Endoscopic Third Ventriculostomy Success Score (ETVSS). This was developed by Kulkarni et al.29). They used logistic regression techniques to predict the success of ETV with relation to age of the patient, etiology for hydrocephalus and previous shunting history. The resulting ETVSS has a range from 0 (very poor success) to 90 (very high success). The score approximates the percent chances of successful outcome at six months following procedure. Age ranges from less than one month, one month to six months, six months to a year, one year to ten years and beyond ten years are given progressively higher scores. Etiology includes post infection, myelomeningocele, intraventricular hemorrhage, non tectal tumors. Aqueduct stenosis and tectal tumors get sequentially better scores. Previous shunt surgery or primary procedure forms the third arm of the scoring system. The final ETVSS is the addition of the age score, etiology score and shunt score. This study included outcomes at 6 months follow up. In a follow up study across all ETVSS groups28), they concluded that as the postsurgical time progresses, the risk of ETV failure reduces in comparison to that of shunt failure. In 2011, the same group of authors also reviewed literature published over 20 years and inferred that the ETVSS can closely predict the actual ETV success rate30).
Age as a determinant
The success rate of ETV in 21 patients less than two years of age was analyzed by Baldauf et al.3). It was found in this study that the success rate of ETV in children less than two years of age suffering from obstructive hydrocephalus depends on age and etiology with an overall success rate of 43%. In infants, ETV was successful in 37.5% of cases. On analyzing ETV done for 41 hydrocephalus patients younger than two years, Sufianov et al.50) observed that ETV was successful in 71.4% of children between one to two years and in 75.0% of children less than one year. They concluded that success of ETV in this group of patients (<two years) depends on the thickness of the floor of third ventricle and the age at which hydrocephalus presented.
He et al.22) have reported sixteen successful ETV procedures done out of seventeen attempted cases of infantile hydrocephalus of varied etiology. A retrospective analysis by Jernigan et al.24) of 5,416 infants with hydrocephalus who underwent CSF diversion either in the form of shunting or ETV observed a failure rate of 64% after ETV, higher than the 40% failure rate seen post shunting. This rate was even more pronounced if ETV was done within 3 months of birth.
Ogiwara et al.39) retrospectively analyzed 23 patients less than six months of age who were treated with ETV. They were of the opinion that ETV can be considered as the primary treatment for hydrocephalus in children above three months of age.
A significant improvement in our understanding has been contributed by the preliminary results published by The International Infant Hydrocephalus Study Group31); This prospective, multicenter comparison of ETV and shunt success in patients less than two years of age has analyzed 158 patients and their results suggest that shunting has a superior success rate as compared to ETV (66% vs. 88% at age of six months).
It seems that ETV has a higher possibility of success than what is described in the ETVSS, especially above the age of 3 months.
Etiology as a determinant
Within the parameters of age, etiology may also play a significant role.
Koch and Wagner26) found poorer results of ETV in cases of obstructive hydrocephalus other than aqueduct stenosis and in very young age group of patients.
In the common scenario prevalent in South Asia and Africa, of post-meningitis hydrocephalus, particularly associated with tuberculosis, several studies have been described for ETV. ETV has been shown to have a success rate between 60–85% in most series published16). ETV helps to divert the CSF to areas which were previously inaccessible and clears exudates from the areas which had impaired absorption, thus helping to improve drug delivery25). Chugh et al.6) suggested in 2009 that ETV can be considered as the primary interventional modality in patients with tuberculous meningitic hydrocephalus especially those with a longstanding disease.
ETV in spina bifida patients more than 6 months of age after shunt failure has been shown to have a good long term success (approximately 80%)52).
ETV in Dandy Walker malformation can be an effective means to achieve reduction in hydrocephalus and is a recommended line of treatment23).
Hydrocephalus in Chiari 1 malformation is a known entity with a complex etiology which is a matter of great debate. However the use of ETV in Chiari 1 malformation is gathering pace mainly because it causes reduced hampering of the physiological pathways of CSF flow and absorption9).
ETV for craniosynostosis has a relatively higher failure rate. Out of eleven patients of craniosynostosis with hydrocephalus treated by Di Rocco et al.10), seven patients were successful in the outcome and shunting was required for the remaining four. A closer monitoring is recommended in these scenarios.
In terms of ETV in tumoral hydrocephalus; in a study of thirty pediatric patients developing hydrocephalus amongst 104 who underwent posterior fossa surgery, ETV was found to have a success rate of more than 90% and has been recommended as the ideal treatment for hydrocephalus in such cases51). ETV for pineal region tumours is regarded as the primary line of intervention with the advantage of not only relieving hydrocephalus but also providing window for biopsy, CSF analysis and to inspect for tumor seedlings and dissemination if any38).
ETV is also shown to have similar if not better results to shunting in cases of normal pressure hydrocephalus and can be recommended as a primary line of treatment19).
Past history of shunting as a determinant
Labidi et al.33) studied the ETVSS in 168 patients more than two years of age recently. They found the ETVSS to be more useful in situations where 60% or 70% success rate is the threshold for preferring ETV to CSF shunt. Past history of shunting and age was not found to be associated with poor outcome unlike the post-hemorrhage and post-infectious causes of hydrocephalus which correlated with reduced success rates post ETV. Melikian and Korshunov37) observed that ETV done in cases of shunt malfunction in obstructive hydrocephalus gave a 70% chance of long term shunt free status.
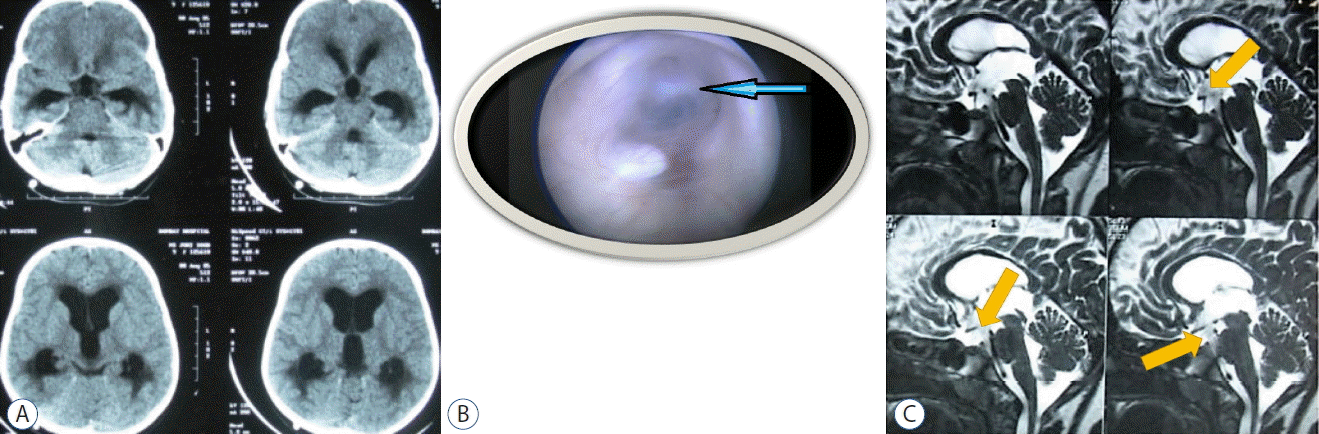
Secondary ETV may be done in the form of repeat endoscopic ventriculostomy for treatment of failed ETV in obstructive hydrocephalus due to stoma closure (Fig. 3A–C). Mahapatra et al.35) in a study of 32 cases of failed ETV who underwent re-ETV suggested that secondary ETV is an effective procedure and can be offered to the patient prior to shunt insertion. Siomin et al.49) reported an increase in ETV efficacy in post-hemorrhagic hydrocephalus from 60.9% for primary ETV to 100% for secondary ETV. This is attributed to better maturation of arachnoid villi over a period of time after recovery from the acute insult.
ETV following shunt malfunction has also been shown to be an effective treatment for shunt failure. Varying reports in the literature cite an ETV success rate of approximately 65–70% in cases of shunt malfunction14,36).
Secondary ETV has been used in shunt infections. Although the procedure does not obviate the need for later shunt implantation, it is shown to delay the subsequent procedures48). When performing a secondary procedure, some studies recommend removal or blockage of the malfunctioning shunt. This is shown to increase the longevity of the procedure.
In our experience, ten cases of shunt malfunction underwent ETV at a mean age of nine years; seventy percent success rate was seen with secondary ETV for a single procedure. Two of the remaining patients responded to repeat ETV while one with patent stoma required shunt insertion. We found that the follow up period required is much longer than for a primary procedure, as failures were seen even two years following secondary ETV.
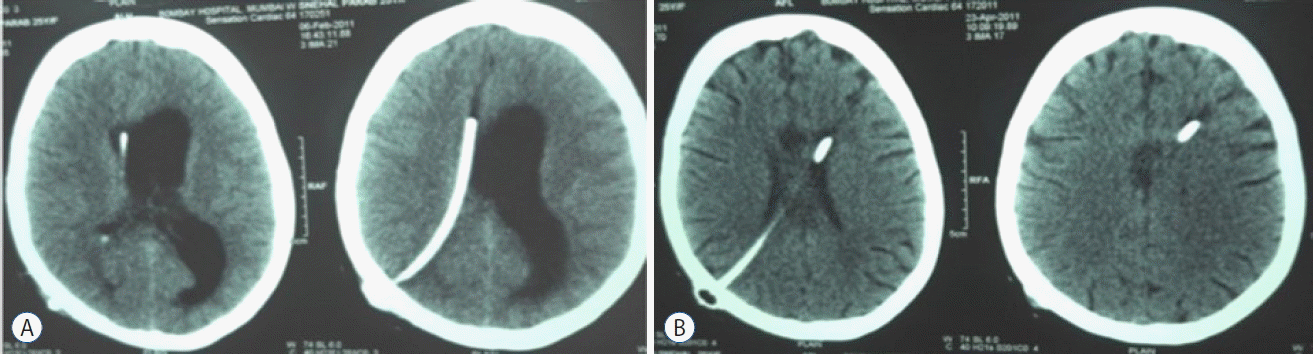
Other endoscopy procedures like septostomy may help a previously shunted patient in cases of unilateral ventricle being drained by shunt due to foraminal block (Fig. 4A, B). Aqueductoplasty has, however, not been popular because of its high morbidity45).
LONG TERM NEUROPSYCHOLOGICAL OUTCOME
A study of thirteen patients with non-communicating hydrocephalus with concurrent cognitive dysfunction by Hader et al.21) showed significant improvement (approximately 70%) in at least one clinical entity post ETV. It has not yet been conclusively proven that shunting gives a better neuropsychological outcome over long term as compared to ETV2). The long term intellectual and psychological outcomes are also currently being evaluated by the International Infant Hydrocephalus Study group31).
VALIDATION OF ETVSS
Various studies have validated this scoring system to predict success of the procedure. Durnford et al.12) externally validated the ETVSS by retrospectively comparing actual success at both 6 and 36 months in 166 patients of a mean age of 39 months. They concluded that the ETVSS closely predicted the overall long term success rates in high, moderate and low risk groups. Breimer et al.4) studied 104 patients of hydrocephalus less than 18 years of age and found the ETVSS to be a more reliable indicator of short term success rather than long term one. Foley et al.17) consider the ETVSS to be an adequate tool for individual assessment of patients and for charting their prospective course with respect to ETV response.
ETV VS. SHUNTING
Tuli et al.53) first showed in 1999 in their study of 242 cases, that results were comparable between shunting and ETV (44% vs. 45%). A multicenter study comprising over 1000 patients by Kulkarni et al.28) has studied this subject in further detail while devising the ETVSS. In the recently published systematic review by Limbrick et al.34) quoting more than 100 articles, evidence suggests that both shunting and ETV provide an equivalent result in most cases of pediatric hydrocephalus.
However shunt may not be the panacea and may have certain contraindications as evidenced by one of our patients recently. A 20 year boy presented to us with history of extrusion of shunts through the skin three times in the past following shunt revision done sixteen times. He was diagnosed as a case of silicon allergy and underwent ETV as no shunt option was left. Obstructive pattern of hydrocephalus was the only predictive factor. No landmarks were seen on the third ventricular floor. Navigation guided ETV was performed. The patient has been clinically stable during the last two years.
CONCLUSION
ETV has further increased our understanding of hydrocephalus in many ways and has been a useful adjunct in management of pediatric hydrocephalus.
With increasing use of this method, the indications are getting refined. The prediction of ETV success and failure is possible today for immediate success and with more experience can be applied as a long term outcome indicator. Though shunting is a useful tool in treatment of hydrocephalus, and is more widely applicable than ETV for pediatric hydrocephalus, it may not be the first choice in selected cases where ETV gives comparable results with the distinct advantage of freedom from hardware and its associated risks.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download