Abstract
Objective
To determine the advantages of parietal approach compared to Kocher's point approach for spontaneous, oval-shaped intracerebral hemorrhage (ICH) with expansion to the parietal region.
Methods
We divided patients into two groups : group A had burr holes in the parietal bone and group B had burr holes at Kocher's point. The hematoma volume, Glasgow coma scale (GCS) score, and modified Barthel Index (mBI) score were calculated. At discharge, we evaluated the patients' Glasgow outcome scale (GOS) score, modified Rankin Scale (mRS) score, motor grade, and hospitalization duration. We evaluated the patients' mBI scores and motor grades at 6 months after surgery.
Results
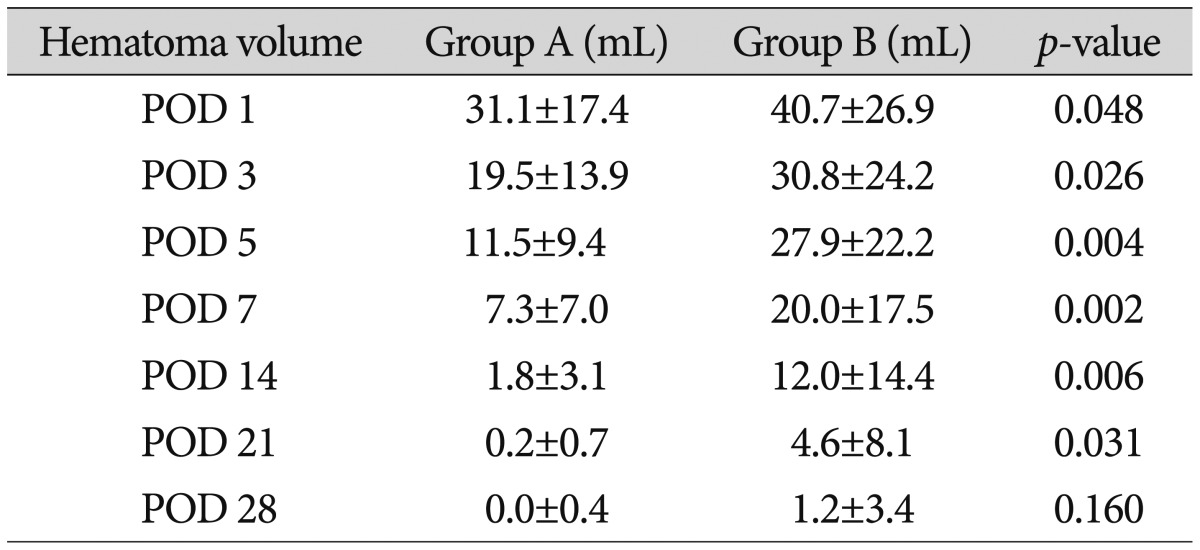
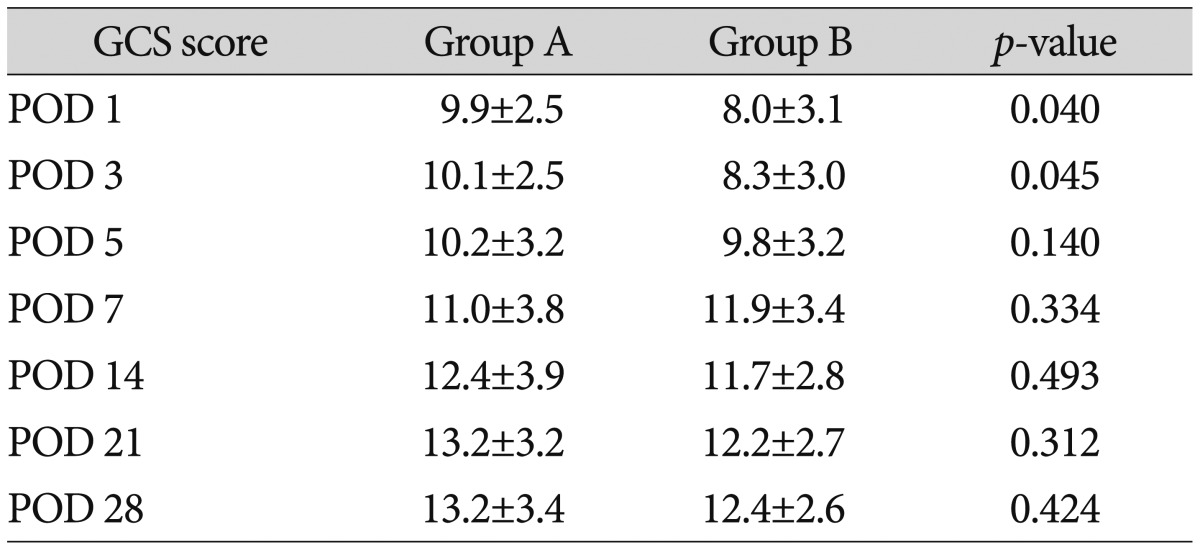
The hematoma volume in group A was significantly less than that in group B on postoperative days 1, 3, 5, 7, 14, and 21. Group A had significantly higher GCS scores than did group B on postoperative days 1 and 3. Group A had higher mBI scores postoperatively than did group B, but the scores were not significantly different. No differences were observed for the GOS score, mRS score, motor grade at discharge, or duration of hospitalization. The mBI score of group A at 6 months after surgery was significantly higher, and more patients in group A showed muscle strength improvement.
Spontaneous intracerebral hemorrhage (ICH) is one of the most common emergency diseases in neurosurgery, with a prevalence of about 10-15% among cerebrovascular accidents, and is associated with high morbidity and mortality rates12368). Regarding treatment, surgery is considered in cases with large hematomas, lack of intracranial pressure control, and worsening neurological symptoms14). The purposes of surgery are to regulate the intracranial pressure by intracranial decompression, prevent secondary damage by improving the blood flow, reduce the permanent neurological deficits, and shorten the length of convalescence.
It showed that the hematoma in ICH of basal ganglia or thalamus was oval shape with parietal expansion as well as sphere shape (Fig. 1). The authors suggested that effective drainage of the hematomas could be achieved by placing a catheter parallel to the major axis during stereotactic surgery of oval-shaped ICH with expansion to the parietal region. However, inserting a catheter through the preexisting burr hole at Kocher's point is difficult, thus we devised a method of making a burr hole in the parietal bone in order to operate on oval-shaped ICH with expansion to the parietal region. The aim of this study was to determine the advantages of using the parietal approach compared to the Kocher's point approach when operating on spontaneous, oval-shaped ICH with expansion to the parietal region.
We studied patients who were admitted to our hospital between January 2009 and December 2011 with a diagnosis of unilateral basal ganglia or thalamic ICH, with oval-shaped hematomas that had expanded to the parietal region, and who had undergone stereotactic hematoma removal surgery with 4-week follow up. Patients with ICH that were due to arteriovenous malformations, aneurysms, Moyamoya disease, hemorrhagic infarctions, or tumor bleeding were excluded. Patients with coagulopathies such as liver cirrhosis or those who were taking anticoagulants were also excluded.
Patients were divided into two groups : group A included patients whose surgery involved making a burr hole in the parietal bone, while group B included patients whose surgery had utilized the Kocher's point approach during the same time and under the same circumstances. The type of surgical approach was chosen at random. Each group was investigated in terms of their sex, age, Glasgow coma scale (GCS) score on admission, time interval from admission to operation, hematoma volume on admission, and operation time. The patients' GCS scores and hematoma volume on postoperative days 1, 3, 5, 7, 14, 21, and 28 were calculated. The patients' modified Barthel Index (mBI) score was obtained on postoperative days 14, 21, and 28. The hematoma volume was measured on brain computed tomography (CT) images, as follows11) : (a×b×c)×0.5, where a and b refer to the diameters of the hematoma with the largest area, while c refers to the thickness. The Glasgow outcome scale (GOS) score, modified Rankin Scale (mRS) score, motor grade on discharge, and duration of hospitalization were examined. We again obtained the patients' mBI scores and motor grades at 6 months after surgery.
We used frame-based stereotactic catheter insertion and drainage with a Leksell stereotactic frame (Leksell® Coordinate Frame G Kit with 10 RFS, ELECTA, Stockholm, Sweden). The patients were in the semi-Fowler's position, with 30° flexion of the head to make the surgery easier.
Two factors were considered when deciding on the burr hole site. First, we considered placing the burr hole posterior to the parietal eminence, as there is a risk of motor cortex injury when placing the burr hole in front of the parietal eminence. Second, we utilized the axial, coronal, and sagittal images of each patient's brain CT to determine the site that would allow the most efficient removal of the hematoma. Once the site had been determined, we performed burr hole trephination (Fig. 2).
The number of catheters used depended on the volume of the hematoma. One catheter was inserted if the volume was less than 30 mL, two catheters were inserted if the volume was 30-59 mL, and three catheters were inserted if the volume was over 60 mL. We applied negative pressure using a syringe placed in the catheter, which was inserted carefully into the hematoma; we aspirated the hematoma for less than 10 mL, and then connected a drainage bag to naturally drain the remaining hematoma. We acquired brain CT images on postoperative days 1, 3, and 5 to withdraw the catheter in order to remove the remaining hematoma, and the catheter was removed on postoperative day 7 (Fig. 3). We injected 3000 U of urokinase when the hematoma drainage was poor for up to 2 days after surgery.
Chi-squared tests and Student's t-tests were performed to identify any statistically significant differences between the groups. A p value of <0.05 was considered statistically significant.
The parietal approach was utilized in 43 patients, although four patients died and one was transferred to another hospital on the 5th postoperative day, thus group A contained 38 patients. The Kocher's point approach was used in 54 patients, but four died, thus group B contained 50 patients.
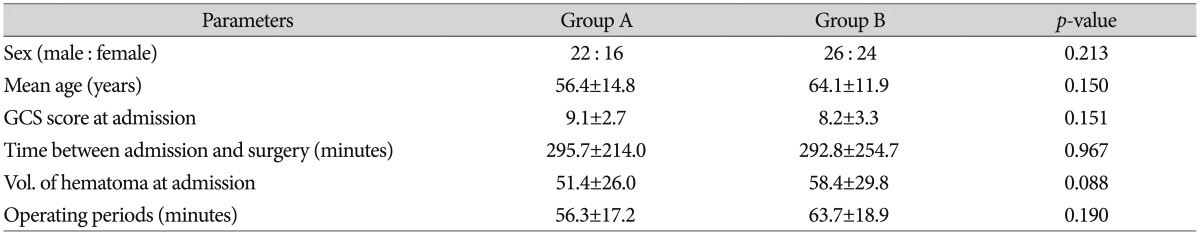
Group A consisted of 22 men and 16 women, and the average age of the patients was 56.4±14.8 years. Group B consisted of 26 men and 24 women, with an average age of 64.1±11.9 years. The GCS scores at admission were 9.1±2.7 and 8.2±3.3 in groups A and B, respectively, and the time intervals between admission and operation were 295.7±214.0 min and 292.8±254.7 min, respectively. The average operation times for groups A and B were 56.3±17.2 min and 63.7±18.9 min, respectively. The hematoma volumes at admission for groups A and B were 51.4±26.0 mL and 58.4±29.8 mL, respectively. None of these measures were significantly different between the groups (Table 1).
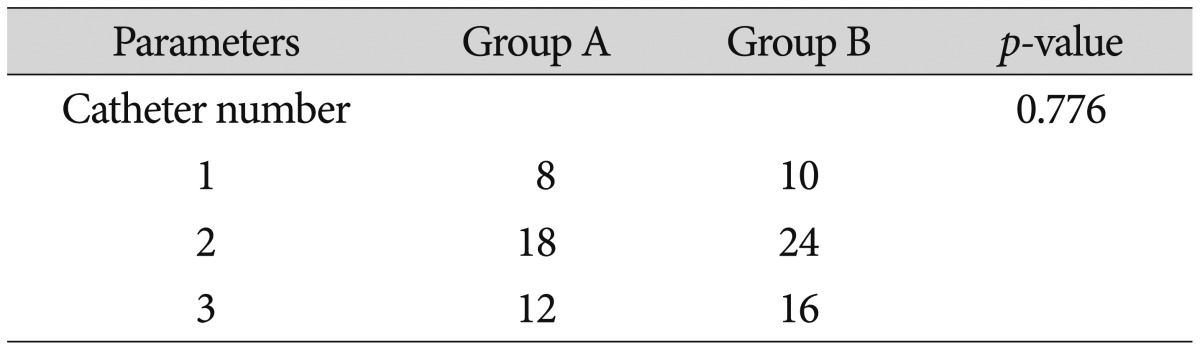
The number of catheters inserted during surgery for group A was one in 8 patients, two in 18 patients, and three in 12 patients. In group B, one catheter was inserted in 10 patients, two in 24 patients, and three in 16 patients. No statistically significant differences were observed between the two groups in terms of the number of catheters inserted (Table 2).
The average hematoma volume on postoperative days 1, 3, 5, 7, 14, 21, and 28 in group A were 31.1±17.4 mL, 19.5±13.8 mL, 11.5±9.4 mL, 7.3±7.0 mL, 1.8±3.0 mL, 0.2±0.7 mL, and 0±0.4 mL, respectively, while those in group B were 40.7±26.9 mL, 30.8± 24.2 mL, 27.9±22.2 mL, 20.0±17.5 mL, 12.2±14.4 mL, 4.6±8.1 mL, and 1.2±3.4 mL, respectively. Less hematoma volume remained undrained in group A compared to in group B. The hematoma volumes between the two groups on postoperative days 1, 3, 5, 7, 14, and 21 were significantly different (Table 3).
The average GCS scores of the patients in group A on postoperative days 1, 3, 5, 7, 14, 21, and 28 were 9.9±2.5, 10.1±2.5, 10.2± 3.2, 11.0±3.8, 12.4±3.9, 13.2±3.2, and 13.2±3.4, respectively, while those of group B were 8.0±3.1, 8.3±3.0, 9.8±3.2, 11.9±3.4, 11.7± 2.8, 12.2±2.7, and 12.4±2.6, respectively. The GCS scores of group A on postoperative days 1 and 3 were significantly higher than the scores of group B (Table 4).
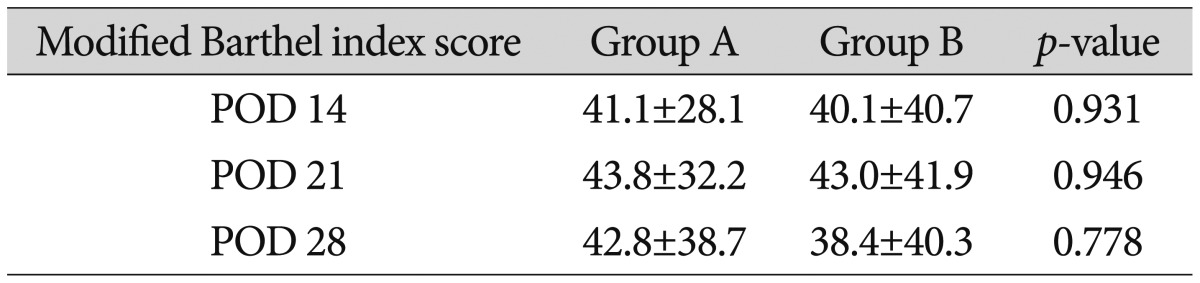
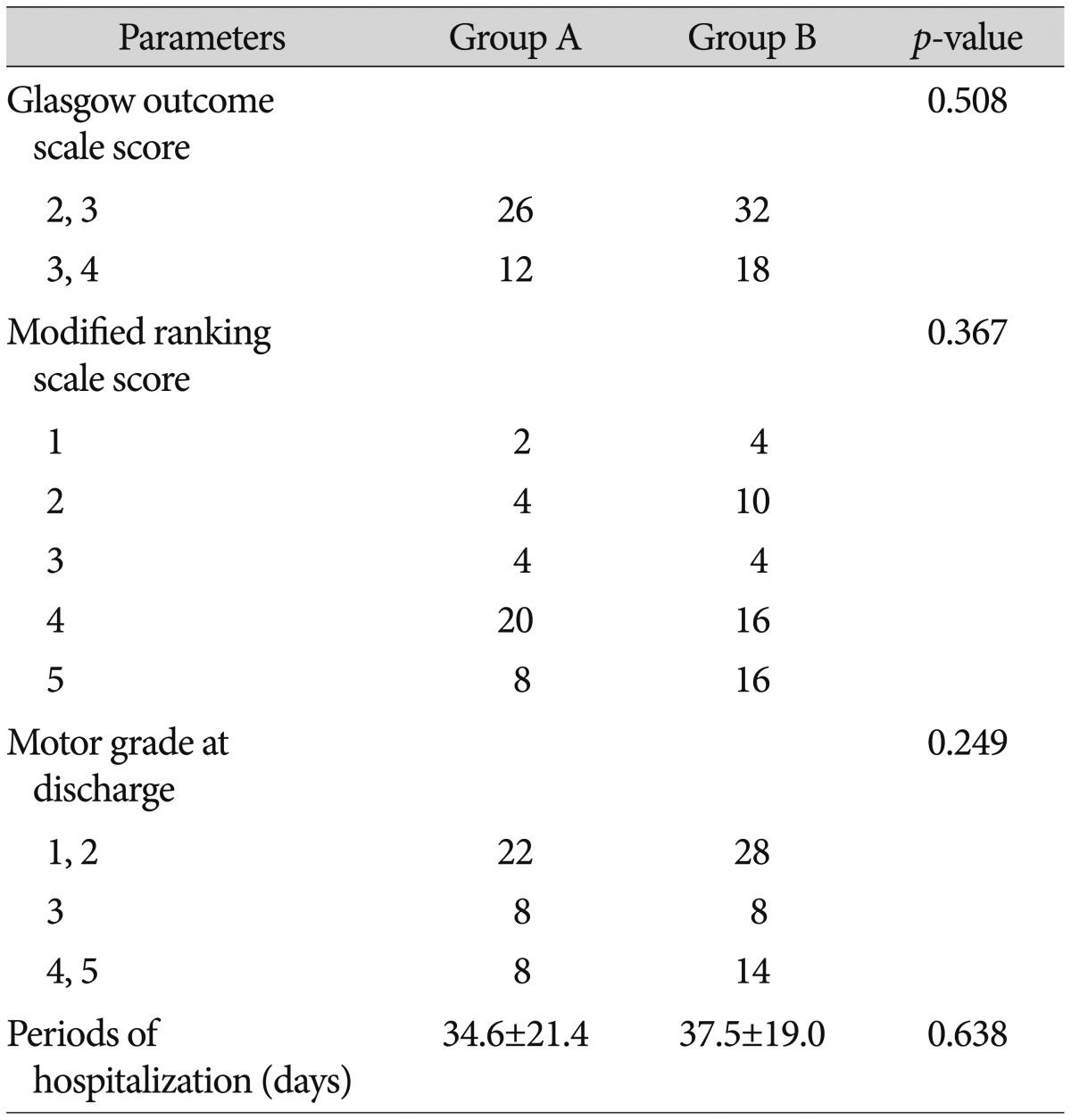
The average mBI scores of patients in group A on postoperative days 14, 21, and 28 were 41.1±28.1, 43.8±32.2, and 42.8±38.7, respectively, while those of group B were 40.1±40.7, 43.0±41.9, and 38.4±40.3, respectively. Although, group A had higher scores than group B, the difference did not reach statistical significance (Table 5). 26 patients from group A and 32 patients from group B had GOS scores of 2-3. 12 patients from group A and 18 patients from group B had scores of 4-5, but the differences were not statistically significant. Neither the mRS scores nor the motor grades at discharge were significantly different between the two groups. The average hospitalization durations were 34.5±21.4 days and 37.4±19.0 days in groups A and B, respectively, and these durations were not significantly different (Table 6).
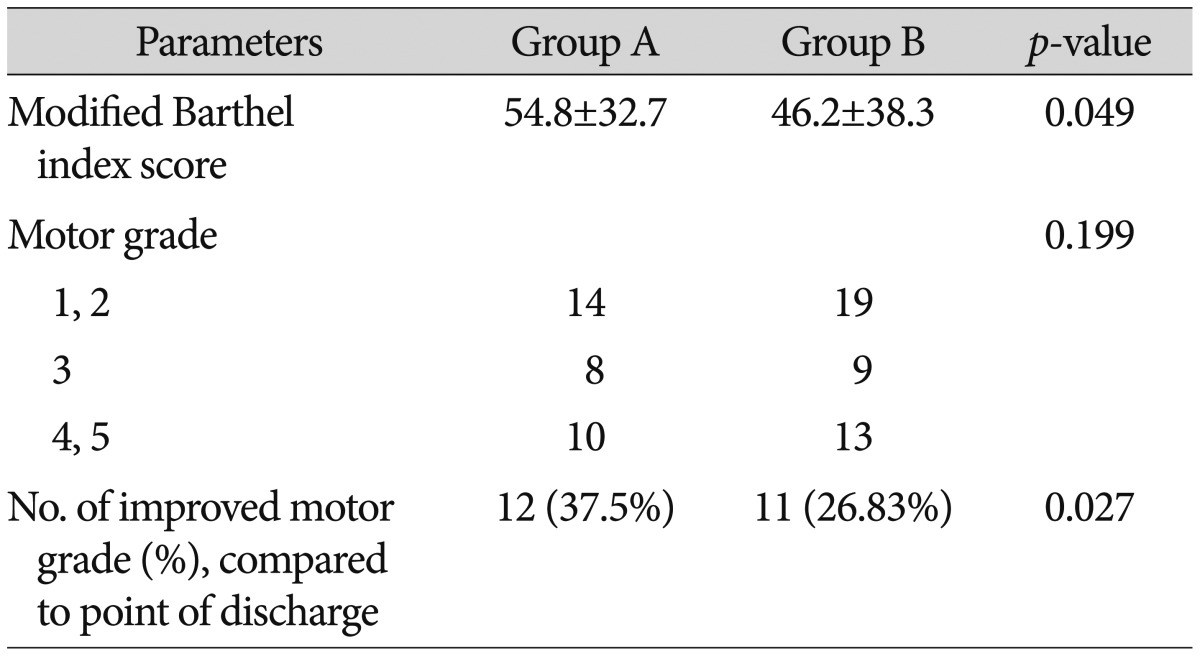
We reevaluated the patients' mBI scores and motor grades at 6 months after surgery. Six of the 38 patients in group A and 9 of the 50 patients in group B left the study before follow-up. The mBI score at 6 months after surgery was 54.8±32.7 in group A and 46.2±38.3 in group B. Group A had significantly higher scores compared to group B. The number of patients with improved muscular strength in group A was 12 (37.5%), while that in group B was 11 (26.83%), and the number in group A was significantly higher than that in group B (Table 7).
Kuo et al.12) reported that the average preoperative GCS score of patients with ICH in the basal ganglia was 7.8, while the average GCS score on endoscopically postoperative day 7 was 12.2. The present study found similar results, with patients demonstrating preoperative GCS scores of 8-9 and postoperative day 7 GCS scores of 11-12.
There were no differences in the operation methods between the parietal approach and Kocher's point approach. However, when using the parietal approach, it was difficult to insert the catheter and create the burr hole while patients were in the supine position. The patient was put in the semi-Fowler's position and the head was flexed about 30 degrees. No respiratory or other problems caused by the head flexion were noted and the operation proceeded uneventfully.
Injuries of the motor cortex or internal capsule, including the corticospinal tract (CST), may cause a permanent disability by operation or by ICH9). Because the posterior limb of the internal capsule is located between the basal ganglia and thalamus, the precise route must be assessed before surgery and should be approached with caution, especially in these areas. In stereotactic surgery via Kocher's point, there is a few risk of injuring the motor cortex or internal capsule, but there are some risks in the parietal approach. Therefore, we placed the burr hole posterior to the motor cortex to avoid injury. That is, we suggest that we alleviated the risk of motor cortex injury by means of placing the burr hole behind the parietal eminence.
The internal capsule may also be identified via intraoperative direct subcortical stimulation in order to avoid causing any damage to the capsule7). Although this may be a concern in some surgeries, such as those for deep brain stimulation, we did not consider using this method here, as it is not conducted in most stereotactic hematoma removals for ICH. The motor cortex and internal capsule areas were confirmed using three-dimensional (3D) brain CT before the operation. The 3D CT is more advantageous than plain CT, as they allow accurate visualization of the hematoma and surrounding structures using coronal and sagittal images, thus reducing injuries to eloquent areas. If the motor cortex and posterior limb of the internal capsule were intact, the location of the burr hole was chosen so that the catheter insertion route avoided these areas. We selected the site of catheter insertion to maximize hematoma removal if there was obvious damage by hematoma not moved back on brain CT image. None of the surgeries resulted in damage to the internal capsule by the catheter according to postoperative brain CT image.
During the acute phase of spontaneous ICH, decreases in the cerebral blood flow (CBF) are observed in the areas around the hematoma because of increased intracranial pressure, mass effects of the hematoma, and changes in microcirculation pressure1516). The CBF of patients who had stereotactic hematoma removal improved in 2/3 of patients, as measured using Xe-133 inhalation and single photon emission computed tomography. Moreover, the decrease in CBF during the acute phase is inversely correlated to the hematoma volume517). Removing the hematoma through surgery not only lowers the intracranial pressure, but also helps improve CBF. In the present study, the preoperative hematoma volumes of the two groups did not differ, and hematomas were not observed at 4 weeks after the operation. Nevertheless, follow-up brain CT scans that were performed at various points after the surgery showed that patients in group A had lower hematoma volumes than did patients in group B, and this difference was statistically significant through postoperative day 21. In addition, the GCS scores after the operation were slightly higher in group A than they were in group B, and the scores obtained on postoperative days 1 and 3 were significantly different between the two groups. Therefore, the parietal approach is considered to be more effective at removing the hematoma in the early stages, thus improving the clinical symptoms at the acute phase.
The mBI score was also measured to evaluate the postoperative clinical symptoms. It was difficult to evaluate the mBI score since the catheter remained inserted for 7 days after the surgery; hence, the mBI score was evaluated on postoperative days 14, 21, and 28. Group A had slightly better scores for daily living activities than did group B. This difference may be because the removal was more effective in group A during the acute phase. Although the difference was not significant, it might be meaningful if there are more cases.
There was a trend towards higher GCS and mBI scores in group A compared to in group B in the acute phase, but the GOS score, mRS score, and motor grades at discharge were not significantly different between the two groups. We suggest they would make no difference practically, as the values are not continuous variables, but rather ranked variables and nominal scales, thus the values would not precisely reflect the clinical prognoses of the two groups.
The number of patients significantly improved muscular strength and mBI scores at 6 months after surgery was higher in group A than it was in group B. The improvement in motor power may have improved the scores for activities of daily living. Motor weakness in basal ganglia ICH stems from injury to or pressure on the CST. So if the mass effect of the hematoma is decreased, then muscular strength would improve along with the recovery of CST pressure4). Additionally, descending motor pathways including non CST (reticulospinal tract, vestibulospinal tract, anterior CST) can compensate for the muscle force of the lower extremities via bypass in CST injury1013). Therefore, the early effective removal of hematomas via the parietal approach may prevent additional CST injuries and help recovery in muscle strength 6 months after surgery.
The limitation of this research is using GCS score, GOS score, mBI score, mRS score. These are subjective measurements, ranking variables, and nominal scales rather than continuous variables. In this study, examinations such as transcranial ultrasonography, perfusion magnetic resonance imaging or monitoring through the intracranial pressure catheter insertion, etc would be more objective and precise assessments.
In oval-shaped ICH with expansion to the parietal region, the parietal approach is considered to improve the clinical symptoms at the acute phase by removing the hematoma more effectively in the early stages. The parietal approach might be beneficial in the long-term recovery of motor power.
References
1. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community : the Oxfordshire Community Stroke Project--1981-86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990; 53:16–22. PMID: 2303826.

2. Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993; 78:188–191. PMID: 8421201.

3. Castellanos M, Leira R, Tejada J, Gil-Peralta A, Dávalos A, Castillo J. Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2005; 76:691–695. PMID: 15834028.

4. Cho SH, Kim SH, Choi BY, Cho SH, Kang JH, Lee CH, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett. 2007; 421:142–146. PMID: 17566651.

5. Cho TG, Nam DH, Cho BM, Lee JI, Kim JS, Hong SC, et al. Stereotactic evacuation of spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc. 1999; 28:237–245.
6. Delgado P, Alvarez-Sabín J, Abilleira S, Santamarina E, Purroy F, Arenillas JF, et al. Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2006; 67:94–98. PMID: 16832084.

7. Duffau H. Intraoperative direct subcortical stimulation for identification of the internal capsule, combined with an image-guided stereotactic system during surgery for basal ganglia lesions. Surg Neurol. 2000; 53:250–254. PMID: 10773257.

8. Fewel ME, Thompson BG Jr, Hoff JT. Spontaneous intracerebral hemorrhage : a review. Neurosurg Focus. 2003; 15:E1. PMID: 15344894.
9. Jang SH. A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation. 2009; 24:279–283. PMID: 19458436.

10. Jang SH. The recovery of walking in stroke patients : a review. Int J Rehabil Res. 2010; 33:285–289. PMID: 20805757.
11. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27:1304–1305. PMID: 8711791.

12. Kuo LT, Chen CM, Li CH, Tsai JC, Chiu HC, Liu LC, et al. Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage : case selection, surgical technique, and long-term results. Neurosurg Focus. 2011; 30:E9. PMID: 21456936.
13. Kwon H, Jang SH. Delayed recovery of gait function in a patient with intracerebral haemorrhage. J Rehabil Med. 2012; 44:378–380. PMID: 22434422.

14. Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas : a prospective randomized study. Surg Neurol. 2006; 66:492–501. discussion 501-502PMID: 17084196.

15. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001; 344:1450–1460. PMID: 11346811.

16. Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. Pharmacologic reduction of mean arterial pressure does not adversely affect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit Care Med. 1999; 27:965–971. PMID: 10362421.

17. Tanizaki Y. Improvement of cerebral blood flow following stereotactic surgery in patients with putaminal haemorrhage. Acta Neurochir (Wien). 1988; 90:103–110. PMID: 3281415.

Fig. 1
Brain computed tomography (CT) scans of a 50-year-old man showing a hematoma in the left basal ganglia. A : Axial CT image showing a round hematoma. B : Coronal CT image showing an oval-shaped, spontaneous ICH and an intraventricular hematoma. C : Sagittal CT image showing an oval-shaped, spontaneous ICH that extends to the parietal lobe.
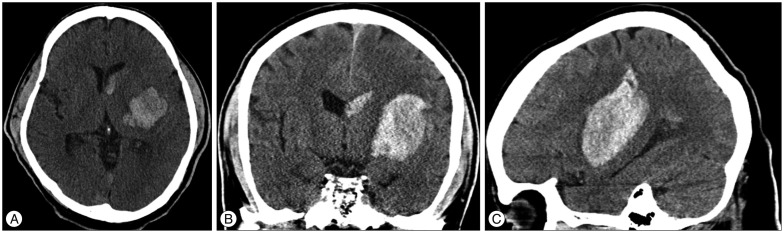
Fig. 2
The burr hole was placed posterior to the parietal eminence so that it would not induce motor cortex injury, and the route and direction of catheter insertion were decided.

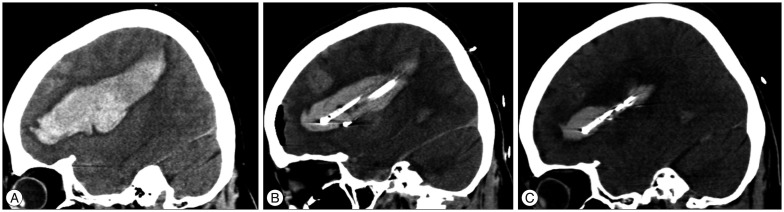
Fig. 3
Pre- and post-operative computed tomography (CT) scans of a 57-year-old female patient with a hematoma that had expanded to the basal ganglia and parietal region. A : Preoperative CT scans in the sagittal plane. B : At postoperative day 1, the CT scans show a decrease in hematoma size and three catheters in the hematoma. C : At postoperative day 3, CT scans show an even smaller hematoma and the two remaining catheters.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download