Abstract
Objective
The occurrence of acute cerebral infection following deep brain stimulation (DBS) is currently being reported with elevation of C-reactive protein (CRP) level. The aim of the present study was to establish normal range of the magnitude and time-course of CRP increases following routine DBS procedures in the absence of clinical and laboratory signs of infection.
Methods
A retrospective evaluation of serial changes of plasma CRP levels in 46 patients undergoing bilateral, two-staged DBS was performed. Because DBS was performed as a two-staged procedure involving; implantation of lead and internal pulse generator (IPG), CRP was measured preoperatively and postoperatively every 2 days until normalization of CRP (post-lead implantation day 2 and 4, post-IPG implantation day 2, 4, and 6).
Results
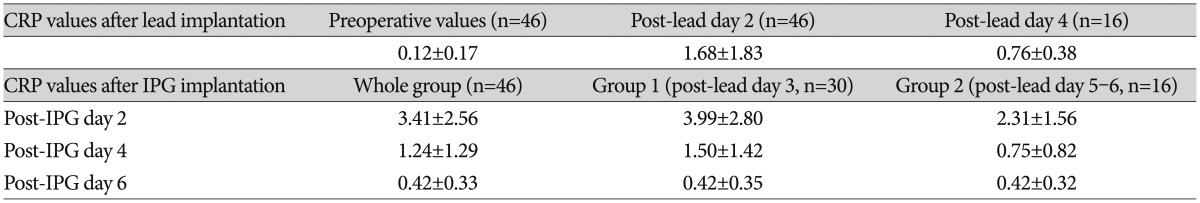
Compared with preoperative CRP levels (0.12±0.17 mg/dL, n=46), mean CRP levels were significantly elevated after lead insertion day 2 and 4 (1.68±1.83 mg/dL, n=46 and 0.76±0.38 mg/dL, n=16, respectively, p<0.001). The mean CRP levels at post-lead implantation day 2 were further elevated at post-IPG implantation day 2 (3.41±2.56 mg/dL, n=46, respectively, p<0.01). This elevation in post-IPG day 2 rapidly declined in day 4 (1.24±1.29 mg/dL, n=46, p<0.05) and normalized to preoperative value at day 6 (0.42±0.33 mg/dL, n=46, p>0.05). Mean CRP levels after IPG implantation were significantly higher in patients whose IPGs were implanted at post-lead day 3 than those at post-lead day 5-6 (3.99±2.80 mg/dL, n=30, and 2.31±1.56 mg/dL, n=16, respectively, p<0.05). However, there was no difference in post-IPG day 2 and 4 between them (p>0.05).
Conclusion
The mean postoperative CRP levels were highest on post-IPG insertion day 2 and decreased rapidly, returning to the normal range on post-IPG implantation day 6. The duration of post-lead implantation period influenced the magnitude of CRP elevation at post-IPG insertion day 2. Information about the normal response of CRP following DBS could help to avoid unnecessary diagnostic and therapeutic efforts.
C-reactive protein (CRP) is an acute-phase protein that is synthesized and rapidly secreted by the liver in response to inflammation, infection, and malignancy624). Repeated examinations of CRP levels in serum is a simple and reliable method for the early detection of bacterial infection and for subsequent monitoring of the response to treatment1012). However, CRP is also elevated after uncomplicated surgery, peaking around the second postoperative day21520), information of the regular postoperative course is necessary when contemplating clinical use of CRP evaluation after neurosurgical interventions115).
In a recent study of surgical site infections (SSI) after deep brain stimulation (DBS)4), CRP was regarded as a poor marker because laboratory findings of elevated CRP were often encountered on the second or third postoperative day after DBS surgery in patients without infection. However, another study5) reported four cases of intracerebral infection occurring within 2-3 days (two patients) and around fourteen days (two patients) after DBS with clinical signs of fever, confusion, and markedly elevated CRP (7.8-16 mg/dL). Therefore, information about normal kinetics of CRP in early postoperative period might have a role as an ancillary test in the early diagnosis of cerebral infection. However, no study has addressed the regular postoperative kinetics of CRP after DBS. The aim of this study was to establish the magnitude and time-course of CRP increases following routine DBS in the absence of clinical and laboratory signs of infections.
From January 2008 to December 2014 we retrospectively evaluated serial changes of plasma CRP levels in 46 patients undergoing two-staged, bilateral DBS procedures among total 65 patients with DBS. The demographics of patients included in this study are summarized in Table 1. Most DBS procedures in the authors' institutions consisted of two procedures. The first was bilateral implantation of the DBS electrode in deep brain nuclei under local anesthesia and externalization of distal lead with several days (3-7 days) of trial stimulation. The second was bilateral implantation of internal pulse generators (IPGs) under general anesthesia23).
Exclusion criteria included all patients in which CRP could be irrelevantly elevated. Hence, we excluded patients who had major surgeries in the last 3 months, patients with chronic inflammatory diseases, underlying liver diseases, and poly-trauma with greater than normal pre-operative CRP levels. In addition, to study normal postoperative kinetics of two-stage, bilateral DBS, we also excluded patients who underwent unilateral DBS (n=6), one stage bilateral DBS in the same day (n=5), those who had revision surgery for misplaced electrode (n=3), patients who had a specific illness following DBS, such as pneumonia or urinary tract infection (n=3), and who had an acute wound infection following DBS were also excluded (n=2). Therefore, during the examination period, 19 patients who did not undergo uneventful, two-stage, bilateral DBS procedures were excluded in this study.
In brief, after taking stereotactic magnetic resonance imaging, the target coordinates calculation and trajectory planning were performed using the commercial planning software (Framelink, ver 5.0, Medtronic, Minneapolis, MN, USA). Under local infiltration of 2% lidocaine, a burr hole trephination and dural opening were performed. After application of stereotactic frame arc with X, Y stages, 3 to 5 trajectories of microelectrode recording (MER) were explored along the planned trajectory to the deep brain nuclei. The location of the final electrode was determined according to microelectrode mapping of the typical firing patterns of MER and microstimulation. The DBS lead (model 3389, Medtronic, Minneapolis, MN, USA) was inserted under the fluoroscopic guidance and test stimulation was performed to assess the improvement of target symptoms and somatosensory side effects. After securing the DBS lead with the burr hole ring and cap, the distal end of the lead was connected to the extension cable through subcutaneous tunneling for external stimulation.
Techniques of transaxillary subpectoral implantation were previously described23). In brief, the patient was placed in the supine position with arms extended and abducted at 90° to expose the axilla. Through 5 cm incision in the prominent skin crease along the axillary floor and subcutaneous dissection, the lateral border of the pectoralis major was searched, and the posterolateral border of the lateral pectoral fascia was entered to gain access to the subpectoral space. With widening of subpectoral pocket by blunt retraction with counter-pulled retractors, subpectoral pocket was generated with gentle finger dissection. Care should be taken not to enlarge the pocket laterally, to limit lateral migration of the implant postoperatively.
Blood samples were collected preoperatively and postoperatively every two days (at days 2 and 4) during external stimulation before implantation of IPGs. After implantation of an IPG, CRPs were again measured every 2 days (post-IPG implantation days 2, 4, and 6) for assessment of serial changes of CRP concentrations. The same surgical team operated on all patients. Although most IPG implantations were performed at days 2-3 after the first procedure, the interval between the first and the second procedures varied in some patients according to assessment of external stimulation results.
To investigate the time course of CRP kinetics, the CRP levels in preoperative state and post-lead implantation day 2 and 4, and post-implantable pulse generator (IPG) implantation day 2, 4, and 6 were compared with paired t-test. To study the influence of the length of external stimulation period on CRP levels, the CRP levels in post-IPG implantation day 2 were subgrouped into those comprising the IPGs were implanted on postoperative day 3 after lead implantation (group 1, n=30), and those IPGs implanted in post-lead insertion 5-6 days (group 2, n=16), and the difference of 2 subgroups were compared independent t-test.
The values and time course of CPR level are summarized in Table 2. CRP levels were measured using a model 7000 clinical analyzer (Hitachi Medical, Tokyo, Japan); the normal range in our institute was 0.01-0.47 mg/dL.
All statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Exploratory analyses of demographics were performed by calculating the means and standard deviations. Statistical differences between the periods (preoperative, post-lead implantation day 2, 4, and post-IPG implantation day 2, 4, and 6) were assessed with paired t-test. Independent t-test was used to investigate the difference of CRP levels in the post-IPG implantation day 2, 4, and 6 according to the external stimulation period (group 1 and 2). Statistical significance was accepted at a probability value of <0.05.
The mean age of the study population was 54.6±16.2 years (range, 15-77 years). Of the 46 patients recruited, 25 were females and 21 were males (Table 1). The mean values and time course of CRP concentrations are summarized in Table 2. The mean CRP concentration was within the normal range before implantation of the leads (0.12±0.17 mg/dL, n=46).
In 46 patients who underwent two-stage DBS, the mean CRP concentration was significantly elevated after lead implantation day 2 (1.68±1.83 mg/dL, n=46, paired t-test, p<0.001). This elevated CRP level in post-lead implantation day 2 decreased in day 4 in group 2 patients (paired t-test, n=16, p<0.05) (Table 2). However, the CRP level in postoperative day 4 was still higher than the preoperative CRP level (paired t-test, n=16, p<0.001). After implantation of bilateral IPGs, the mean CRP concentration rose significantly compared to that of preoperative level (post-IPG day 2, 3.41±2.56 mg/dL, n=46, and 0.12±0.17 mg/dL, repectively. paired t-test, n=46, p<0.05) and those of post-lead implantation day 2 (1.68±1.83 mg/dL, paired t-test, n=46, p<0.05) and day 4 (0.76±0.38 mg/dL, paired t-test, n=16, p<0.05). The mean CRP level was highest on post-IPG day 2 and decreased significantly on post-IPG days 4 and 6 (paired t-test, n=46, p<0.05) and normalized to preoperative value at post-IPG day 6 (paired t-test, n=46, p>0.05).
There was significant difference in CRP level in post-IPG days 2 according to the subgroup analysis of the length of external stimulation period [IPG implantation at post-lead day 3 (group 1) and IPG implantation at post-lead 5-6 (group 2), independent t-test, p<0.05]. However, no difference was found between group 1 and 2 in post-IPG days 4 and days 6 (independent t-test, p>0.05) (Table 2). The differences and time course of CRP values are depicted in Fig. 1.
Infections following DBS are usually found at the site of IPG, at the connector site or on the scalp where the lead exits the brain14). Intracerebral infections following DBS are rare3457911192225), but exist5). To our knowledge, there have been seven reported cases of intracerebral infection related to the implanted electrodes (Table 3). Although diagnosis of deep cerebral infection cannot be established by simple laboratory examination of CRP, marked elevation of CRP values have been reported by Blomstedt and Bjartmarz5) They claimed an intracerebral bacterial infection should be suspected when DBS-implanted patient presents neurological symptoms, fever, peri-electrode edema on CT scan, and elevated CRP. Accordingly, we studied the magnitude and time-course of CRP increases following routine DBS procedures in the absence of clinical and laboratory signs of infections.
CRP is a well-known acute-phase serum protein that increases within 6 hours in response to inflammatory cytokines (mostly interleukin-6)1721). In our study, we defined a value of less than 0.5 mg/dL to be normal and not indicative of inflammation or infection. CRP has been studied as a clinical indicator for the degree of surgical trauma incurred after standard neurosurgical procedures1213151618). The mean postoperative CRP levels are highest in the most surgically traumatic procedure; lobectomy for epilepsy, and lowest in a less surgically-traumatic procedure, stereotactic biopsy, (p<0.001)1). Intraoperative blood transfusion, type of anesthesia, use of anti-inflammatory and antibiotics do not affect CRP kinetics1). The authors of CRP studies115) reported that peak CRP concentration occurred on the 2nd postoperative day in all neurosurgical procedures that included craniotomy for intracranial tumor, aneurysm clipping, intracranial hemorrhage, discectomy with laminectomy, and stereotactic biopsy1). As a potential warning sign for infection, prolonged CRP elevation after the 4th postoperative day or when a second rise in CRP concentration has been suggested12).
Compared with preoperative values, the level of CRP rose significantly after implantation of bilateral intracranial electrodes (p<0.05). The magnitude of CRP elevation after DBS lead insertion at 2nd postoperative day (1.68±1.83 mg/dL) appeared to be less than those reported after stereotactic brain biopsy (5.99±7.1 mg/dL) by Al-Jabi and El-Shawarby1). Although a direct comparison between the present and prior studies is impossible because of differing clinical situations and examination methods, this data might indicate that the magnitude of physiological tissue trauma of DBS lead implantation may be small and comparable to stereotactic biopsy, the least surgically-traumatic, routine neurosurgical procedure1).
After implantation of IPGs, CRP concentration reached its peak at the 2nd postoperative day and rapidly declined at the postoperative days 4 and 6. Compared with mean CRP levels of preoperative and post-lead implantation, elevation of CRP at the 2nd postoperative day after IPG implantation was significant (p<0.05) as was the decline at postoperative day 4 (p<0.05). Therefore, the finding of peak elevation of CRP concentration at postoperative day 2 and the subsequent rapid declines is consistent with previous studies of postoperative CRP kinetics in neurosurgical operations1215). It seems that the CRP concentration routinely rises according to the surgical procedure and remain elevated unitl postoperative day 2-3, after which the level rapidly declines to preoperative levels by postoperative day 4 and 6.
Our study has several limitations. The number of patients recruited in our study is too small and, therefore, there is a risk of type I error to draw a general conclusion about the normal, postoperative CRP kinetics. To overcome this limitation, a more extensive study including more patients with DBS operation is warranted. Because routine DBS procedures in the authors' institution involved a two-stage procedure, the mean values of postoperative CRP may be different in one-staged DBS operation. We also externalized the distal leads and routinely assessed the acute stimulation effect following bilateral DBS. A staged procedure with externalization of the distal lead might influence the CRP levels, given the report of a greater risk of infection following DBS by Constantoyannis et al.8).
Our study involved only the acute postoperative period (until 6 days after IPG implantation). Since the infection rate during this period is quite low, it can be argued that CRP examination following routine DBS would not be helpful. We do not suggest CRP measurement in a routine examination. However, because acute cerebral infection does occur in the immediate postoperative period (less than 2 weeks)45) and we also experienced an acute cerebritis following DBS (not yet reported), we think that knowledge about normal CRP kinetics following routine DBS is helpful in caring for patients with an altered conscious state or vague symptom of headache, which might herald an imminent infection.
Mean plasma CRP level increased significantly after lead and IPG implantation and the elevated CRP level showed a peak concentration at post-IPG implantation day 2, and rapidly decreased in day 4 and day 6. Information about the normal kinetics of CRP levels following DBS would provide reference values and further study including comparison with elevated CRPs in patients with intracerebral infection would be warranted.
References
1. Al-Jabi Y, El-Shawarby A. Value of C-reactive protein after neurosurgery : a prospective study. Br J Neurosurg. 2010; 24:653–659. PMID: 21070150.
2. Bengzon J, Grubb A, Bune A, Hellström K, Lindström V, Brandt L. C-reactive protein levels following standard neurosurgical procedures. Acta Neurochir (Wien). 2003; 145:667–670. discussion 670-671PMID: 14520546.

3. Bhatia S, Oh M, Whiting T, Quigley M, Whiting D. Surgical complications of deep brain stimulation. A longitudinal single surgeon, single institution study. Stereotact Funct Neurosurg. 2008; 86:367–372. PMID: 19033705.
4. Bjerknes S, Skogseid IM, Sæhle T, Dietrichs E, Toft M. Surgical site infections after deep brain stimulation surgery : frequency, characteristics and management in a 10-year period. PLoS One. 2014; 9:e105288. PMID: 25122445.
5. Blomstedt P, Bjartmarz H. Intracerebral infections as a complication of deep brain stimulation. Stereotact Funct Neurosurg. 2012; 90:92–96. PMID: 22353734.

6. Carr WP. The role of the laboratory in rheumatology. Acute-phase proteins. Clin Rheum Dis. 1983; 9:227–239. PMID: 6191909.
7. Chou YC, Lin SZ, Hsieh WA, Lin SH, Lee CC, Hsin YL, et al. Surgical and hardware complications in subthalamic nucleus deep brain stimulation. J Clin Neurosci. 2007; 14:643–649. PMID: 17532500.

8. Constantoyannis C, Berk C, Honey CR, Mendez I, Brownstone RM. Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci. 2005; 32:194–200. PMID: 16018154.

9. Deligny C, Drapier S, Verin M, Lajat Y, Raoul S, Damier P. Bilateral subthalamotomy through dbs electrodes : a rescue option for device-related infection. Neurology. 2009; 73:1243–1244. PMID: 19822876.

10. Du Clos TW, Mold C. The role of C-reactive protein in the resolution of bacterial infection. Curr Opin Infect Dis. 2001; 14:289–293. PMID: 11964845.

11. Falowski S, Ooi YC, Smith A, Verhargen Metman L, Bakay RA. An evaluation of hardware and surgical complications with deep brain stimulation based on diagnosis and lead location. Stereotact Funct Neurosurg. 2012; 90:173–180. PMID: 22678355.

12. Kindmark CO. Quantitative measurement of C-reactive protein in serum. Clin Chim Acta. 1969; 26:95–98. PMID: 5356609.

13. Kratz A, Lee-Lewandrowski E, Lewandowski K. The plasma proteins. In : Lewandrowski K, editor. Clinical Chemistry : Laboratory Management and Clinical Correlations. Philadelphia: Lippincott, Williams and Wilkins;2002. p. 531–560.
14. Merello M, Cammarota A, Leiguarda R, Pikielny R. Delayed intracerebral electrode infection after bilateral STN implantation for Parkinson's disease. Case report. Mov Disord. 2001; 16:168–170. PMID: 11215583.

15. Mirzayan MJ, Gharabaghi A, Samii M, Tatagiba M, Krauss JK, Rosahl SK. Response of C-reactive protein after craniotomy for microsurgery of intracranial tumors. Neurosurgery. 2007; 60:621–625. discussion 625PMID: 17415198.

16. Mustard RA Jr, Bohnen JM, Haseeb S, Kasina R. C-reactive protein levels predict postoperative septic complications. Arch Surg. 1987; 122:69–73. PMID: 3800652.

17. Nathan BR, Scheld WM. The potential roles of C-reactive protein and procalcitonin concentrations in the serum and cerebrospinal fluid in the diagnosis of bacterial meningitis. Curr Clin Top Infect Dis. 2002; 22:155–165. PMID: 12520652.
18. Orriss DE. Serial serum C-reactive protein levels as an indicator of infection in cardiac transplant patients. Med Lab Sci. 1988; 45:116–120. PMID: 3062304.
19. Pepper J, Zrinzo L, Mirza B, Foltynie T, Limousin P, Hariz M. The risk of hardware infection in deep brain stimulation surgery is greater at impulse generator replacement than at the primary procedure. Stereotact Funct Neurosurg. 2013; 91:56–65. PMID: 23207787.

20. Rosahl SK, Gharabaghi A, Zink PM, Samii M. Monitoring of blood parameters following anterior cervical fusion. J Neurosurg. 2000; 92(2 Suppl):169–174. PMID: 10763687.

21. Schuhmann MU, Ostrowski KR, Draper EJ, Chu JW, Ham SD, Sood S, et al. The value of C-reactive protein in the management of shunt infections. J Neurosurg. 2005; 103(3 Suppl):223–230. PMID: 16238075.

22. Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections : incidence and management in a large series. Neurosurgery. 2008; 62:360–366. discussion 366-367PMID: 18382313.
23. Son BC, Han SH, Choi YS, Kim HS, Kim MC, Yang SH, et al. Transaxillary subpectoral implantation of implantable pulse generator for deep brain stimulation. Neuromodulation. 2012; 15:260–266. discussion 266PMID: 22300254.

24. Tillett WS, Francis T Jr. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930; 52:561–571. PMID: 19869788.

25. Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001; 56:548–551. PMID: 11222806.

Fig. 1
An error-box chart showing the mean CRP course of the 46 patients with a rapid increase in the mean CRP value reaching the peak on the second post-IPG implantation day and declining by the 6th postoperative day. *Statistical significance between groups (p<0.05, paired t-test) and the dotted line indicates the upper limit of normal range of CRP (0.01-0.47 mg/dL). CRP : C-reactive protein, PG : implantable pulse generator.
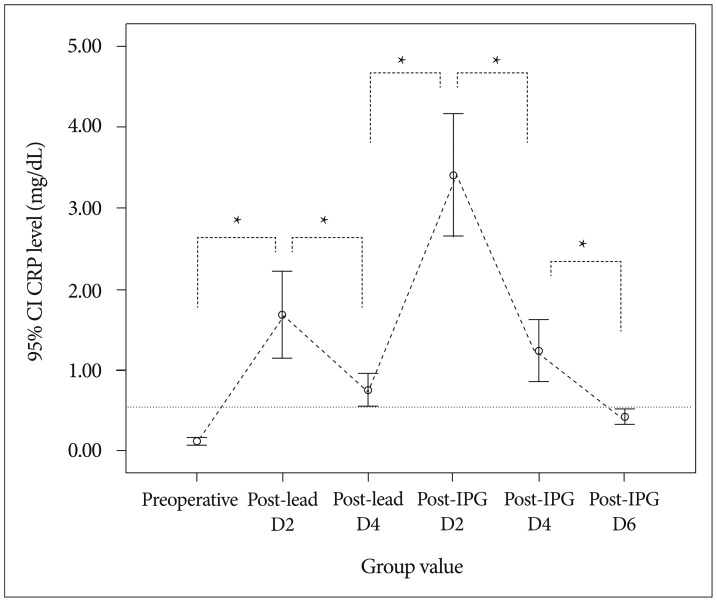
Table 3
CRP values in reported cases of intracerebral infections following DBS
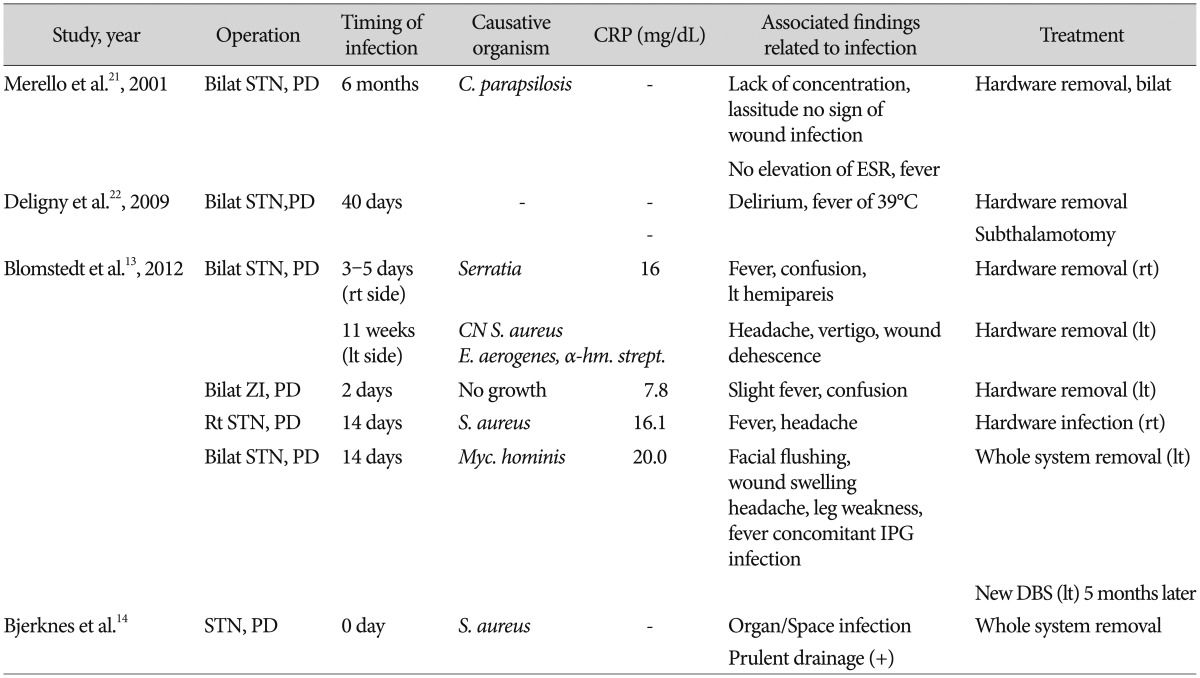
Bilat : bilateral, rt : right, lt : left, PD : Parkinson's disease, ZI : zona incerta, C. parapsilosis : Candida parapsilosis, CN S. aureus : coagulase-negative Staphylococcus aureus, E. aerogenes : Enterobactor aerogenes, α-hemolytic strept. : α-hemolytic streptococcus, IPG : internal pulse generator




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download