Abstract
Objective
Methods
Results
References
Fig. 1
Immunohistochemical analysis of expression of AQP4 in brain tissue of pups from control group (×400). Arrow indicates the AQP4 positive cell. AQP4 : aquaporin-4.
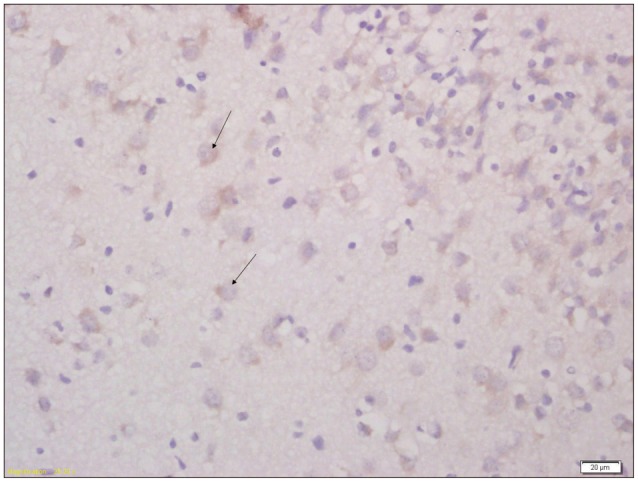
Fig. 2
Immunohistochemical analysis of expression of AQP4 in brain tissue of pups from consolidate group (×400). Arrow indicates the AQP4 positive cell. AQP4 : aquaporin-4.
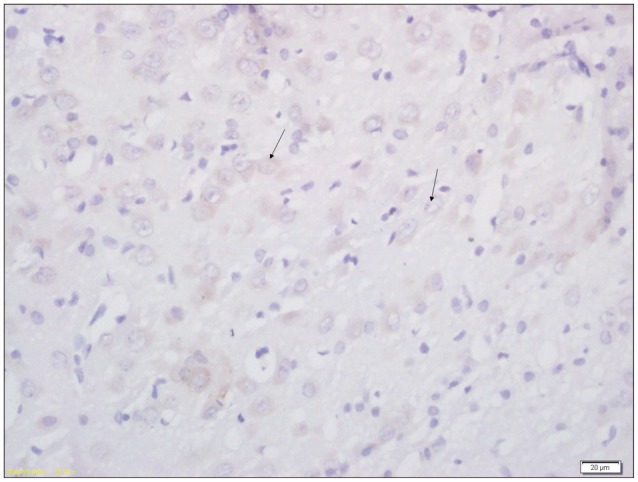
Fig. 3
Immunohistochemical analysis of expression of AQP4 in brain tissue of pups from EI&R group (×400). Arrow indicates the AQP4 positive cell. AQP4 : aquaporin-4.
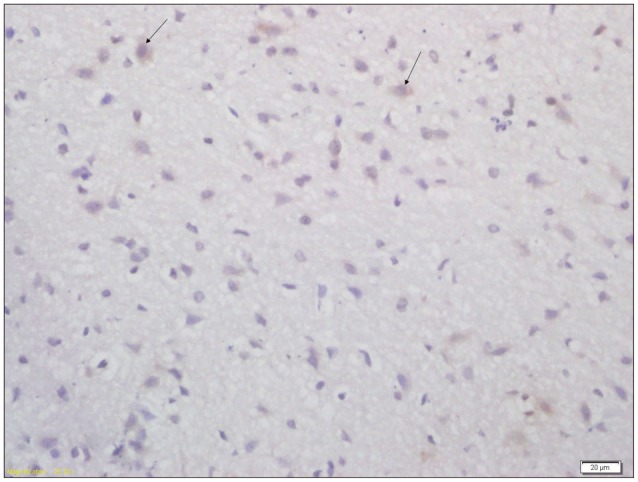
Fig. 4
Immunohistochemical analysis of expression of AQP4 in brain tissue of pups from acupuncture group (×400). Arrow indicates the AQP4 positive cell. AQP4 : aquaporin-4.
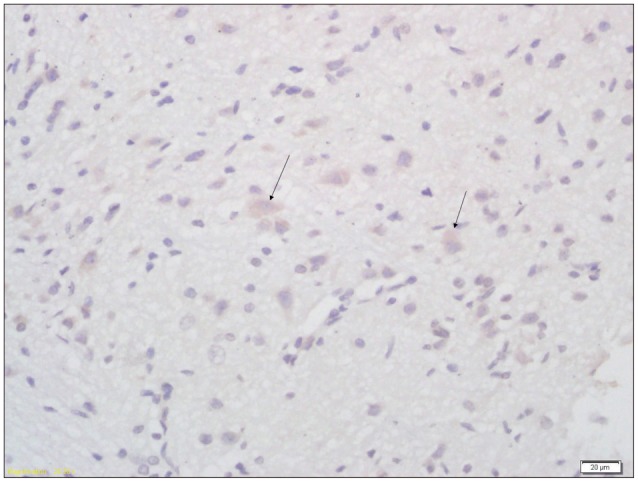
Fig. 5
Immunohistochemical analysis of expression of AQP4 in brain tissue of pups from non treatment group (×400). Arrow indicates the AQP4 positive cell. AQP4 : aquaporin-4.
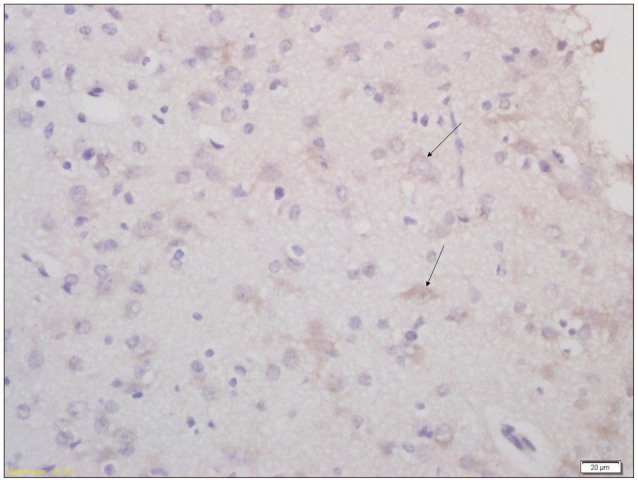
Fig. 6
Western blot analysis of the expression of AQP4 in brain tissue of pups from different groups. Expression of AQP-4 protein is slightly decreased in consolidate group, further decreased in EI&R group and acupuncture group. The expression is markedly decreased in non-intervention group. Lane A : Control group, Lane B : Consolidate group, Lane C : EI&R group, Lane D : Acupuncture group, Lane E : Non-intervention group. AQP4 : aquaporin-4.
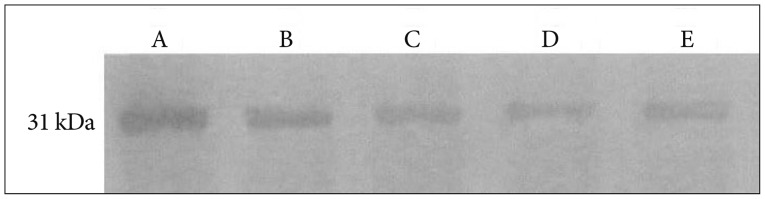
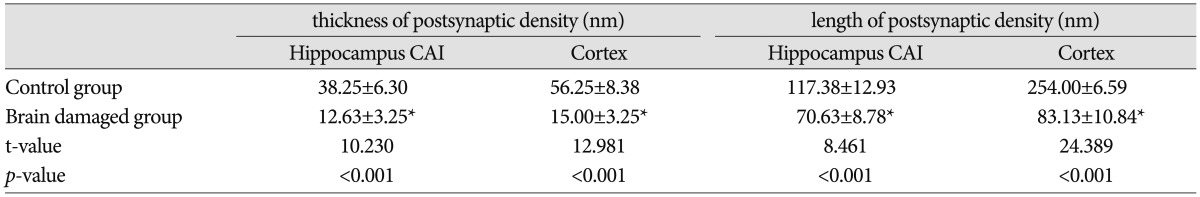
Fig. 7
A : Ultra structure study of hippocampus CAl of pups from the control group. B : Ultra structure study of cortex of pups from the control group. In A and B the structure of synapses is clear and intact, many synaptic vesicles were seen in the presynaptic membrane, the postsynaptic density was thick (black arrow). C : Ultra structure study of hippocampus CAl of the brain damaged group. D : Ultra structure study of cortex of the brain damaged group. In C and D the structural of synapses is unclear, the neurons and synapses were significantly reduced, and also the thickness and the activity length of postsynaptic density reduced, the synaptic vesicles were dissolved and became vacuolize (black arrow). CAl : cornus ammonis I.
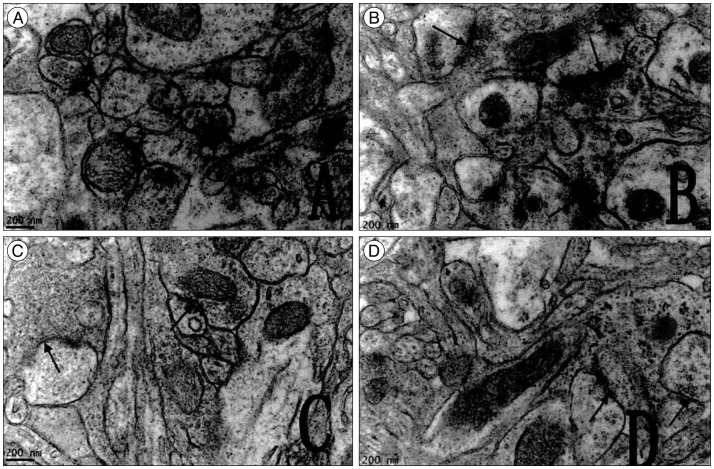
Table 3
Immunohistochemical analysis of expression of AQP4 in brain tissue of pups from different groups
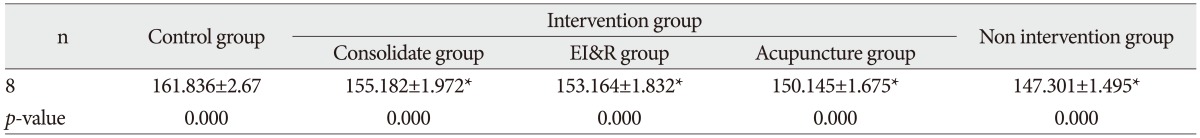
All data are expressed as mean±SD. The results were analyzed by one way analysis of variance followed by t-test for multiple comparisons. There were significantly higher expression of AQP4 in pups from control group in comparison to intervention and non-intervention group (p<0.01) and higher expression in intervention group in comparison to non-intervention group (p<0.01). *p<0.01, compared with each individual group. AQP4 : aquaporin-4
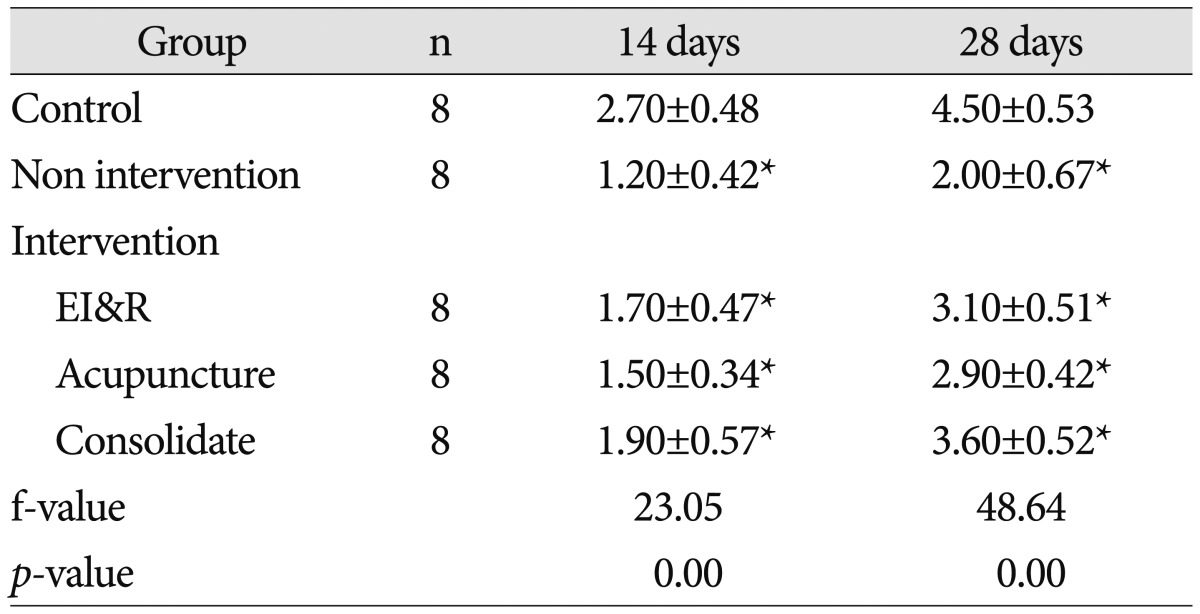
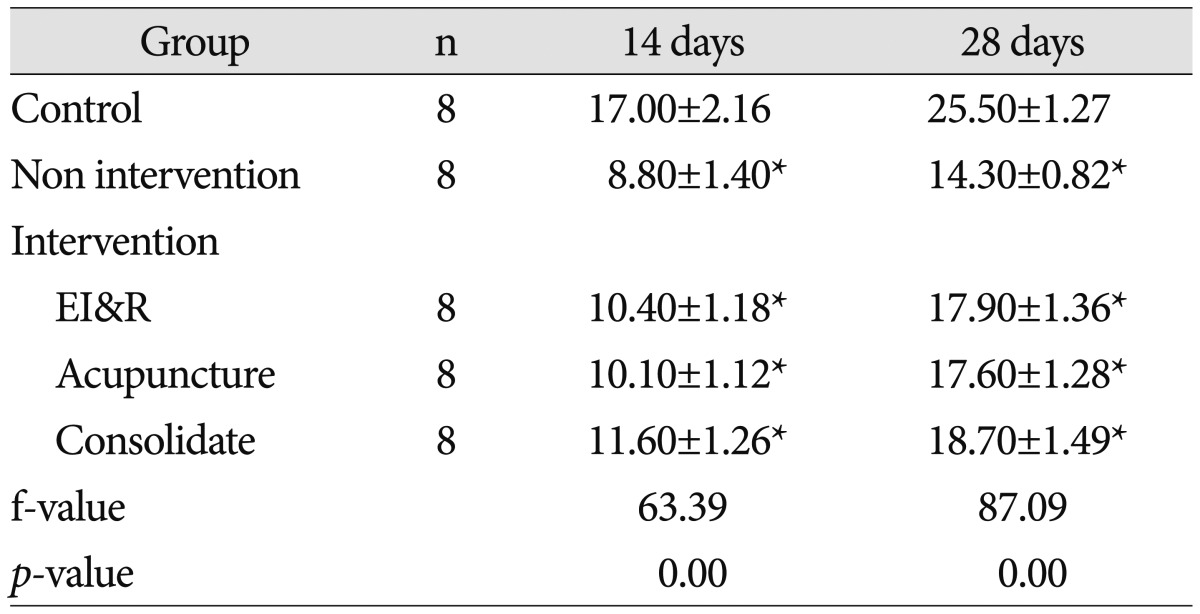
Table 4
Western blot analysis of level of AQP4 expression in brain tissue of pups from different groups
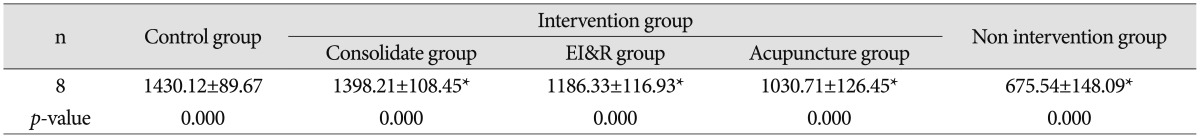
All data are expressed as mean±SD. The results were analyzed by one way analysis of variance followed by t-test for multiple comparisons. There were significantly higher level of AQP4 in control group in comparison to intervention and non-intervention group (p<0.01) and higher level in intervention group in comparison to non-intervention group (p<0.01). *p<0.01, compared with each individual group. AQP4 : aquaporin-4




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download