Abstract
Treatment of Leptomeningeal carcinomatosis (LMC) from solid cancers has not advanced noticeably since the introduction of intra-cerebrospinal fluid (CSF) chemotherapy in the 1970's. The marginal survival benefit and difficulty of intrathecal chemotherapy injection has hindered its wide spread use. Even after the introduction of intraventricular chemotherapy with Ommaya reservoir, frequent development of CSF flow disturbance, manifested as increased intracranial pressure (ICP), made injected drug to be distributed unevenly and thus, the therapy became ineffective. Systemic chemotherapy for LMC has been limited as effective CSF concentration can hardly be achieved except high dose methotrexate (MTX) intravenous administration. However, the introduction of small molecular weight target inhibitors for primary cancer treatment has changed the old concept of 'blood-brain barrier' as the ultimate barrier to systemically administered drugs. Conventional oral administration achieves an effective concentration at the nanomolar level. Furthermore, many studies report that a combined treatment of target inhibitor and intra-CSF chemotherapy significantly prolongs patient survival. Ventriculolumbar perfusion (VLP) chemotherapy has sought to increase drug delivery to the subarachnoid CSF space even in patients with disturbed CSF flow. Recently authors performed phase 1 and 2 clinical trial of VLP chemotherapy with MTX, and 3/4th of patients with increased ICP got controlled ICP and the survival was prolonged. Further trials are required with newly available drugs for CSF chemotherapy. Additionally, new LMC biologic/pharmacodynamic markers for early diagnosis and monitoring of the treatment response are to be identified with the help of advanced molecular biology techniques.
Leptomeningeal carcinomatosis (LMC) is a dismal terminal stage disease of solid cancer that is devastating to the patient. Overall survival (OS) of patients with LMC is approximately 6-8 weeks, and intra-cerebrospinal fluid(CSF) chemotherapy show a survival benefit ranging from 3-9 months according to clinical variables153466).
Problems of intra-CSF chemotherapy include marginal survival benefit over systemic chemotherapy, difficulty of repeated drug injection, poor symptom improvement, and the occurrence of rare but serious side effects such as encephalopathy121319). Among these, CSF flow disturbance, manifest as increased intracranial pressure (ICP) and/or hydrocephalus, is not only a poor prognostic factor but also one of the reasons for treatment failure as it prevents even distribution of injected drugs within the CSF space183237). Research from the 1990s report that CSF flow disturbance is found in 40-60% of patients with LMC based on radionuclide cisternography2132). Once-disrupted CSF flow hardly recovers and increases the risk of encephalopathy via transependymal drug concentration gradient. Most patients quit chemotherapy to selectively receive a ventriculo-peritoneal shunt11618). Reported clinical studies are mainly retrospective, with a small number of patients, limited number of drug injection, and heterogeneous primary cancers. It is therefore difficult to draw any decisive conclusion about the effectiveness of intra-CSF chemotherapy.233850516566).
Chemotherapy for LMC is not curative but palliative. However, studies reporting the response of LMC-related symptoms are rare and the criteria for improvement are subjective385054). Some studies suggested that CSF profiles can be prognostic indicators, but show inconsistent findings of CSF profile change as a treatment response14233766). Another difficulty in evaluating the LMC treatment response is both, the inconsistency of CSF cytology and lack of quantitative measurement of the disease2566). Chamberlain and Kormanik17) suggest a criteria for CSF cytology response of 'two consecutive negative finding at least 1 week apart and sustained for 1 month' but fail to correlate patient survival and reach a general consensus.
It is difficult to estimate the number of clinicians and the frequency at which they apply intra-CSF chemotherapy in patients with poor prognosis, systemic cancer burden, and poor performance profiles. Patients need surgery for subcutaneous intraventricular reservoir for stable drug injection, and require evaluation of physiologic CSF flow and monitoring of drug concentration to avoid toxicities. Despite these difficulties, more aggressive intra-CSF chemotherapy prolongs patient survival in studies of one primary cancer with multiple drug injection and/or salvage intra-CSF chemotherapy172334).
Recently, there are two new trials in the treatment of LMC from solid cancer. First is the increasing application of receptor tyrosine kinase inhibitor (RTKi) for primary cancers, such as epidermal growth factor receptor (EGFR) inhibitor and HER-2 monoclonal antibody in addition to conventional intra-CSF chemotherapy. Prolonged survival is clinically observed in patients who receive RTKi concomitant with intra-CSF chemotherapy. The second is clinical trial of ventriculolumbar perfusion (VLP)3536). VLP is expected to circumvent ineffective drug delivery in LMC patients with disturbed CSF flow but was halted due to technical complexities2449). Authors successfully finished VLP phase 1 with pharmacokinetic data and the following phase 2 trial proved the effectiveness of VLP in LMC patients with increased ICP and prolonged patient survival3536).
Treatments for LMC should cover the whole-neuraxis as the disease spreads. In this context, even whole brain radiation therapy (WBRT) is not an appropriate therapeutic modality for LMC, as it does not cover the entire CSF space and besides, cancer cells move along with the CSF flow. Recent retrospective study on 125 non-small cell lung cancer (NSCLC) patients with LMC demonstrates a lack of effectiveness of WBRT on patient survival48). Seven of these patients with intrathecal chemotherapy show significantly prolonged survival.
Meanwhile, either systemic or intrathecal chemotherapy achieves even distribution of drugs throughout the neuraxis. Pharmacokinetic studies with animal models done in the 1970's have predicted CSF levels of methotrexate (MTX) with clearance rate i.e., 3-5% of systemically administered MTX penetrates the blood-brain barrier (BBB) at a half-clearance time of 6 hours. Thus, to achieve a therapeutic concentration (>1 µM) of MTX in the CSF, grams of MTX are required to be administered intravenously for days5561). Single intraventricular injection of MTX (6.25 mg/m2) can achieve therapeutic concentration in the lumbar space for 48 hours at a minimal systemic absorption (<0.1 µM). Additionally, use of intraventricular Ommaya reservoir has the advantage of patient comfort especially in repeated frequent injection, prevention of drug leakage, and better drug distribution as compared to the intrathecal (via lumbar puncture) chemotherapy59). Thus, studies with repeated aggressive intra-CSF chemotherapy mostly adopt the Ommaya reservoir for intraventricular chemotherapy.
Two prospective studies on the survival of breast cancer patients with LMC compare systemic chemotherapy and radiation therapy with additional intra-CSF chemotherapy1213). Both studies report that intra-CSF chemotherapy does not prolong patient survival and significantly increases associated neurotoxicities. However, in both studies, systemic chemotherapy is not clearly defined, but described as 'appropriate chemotherapy'. Furthermore, ill-defined delayed neurotoxicity was unable to be differentiated from disease progression.
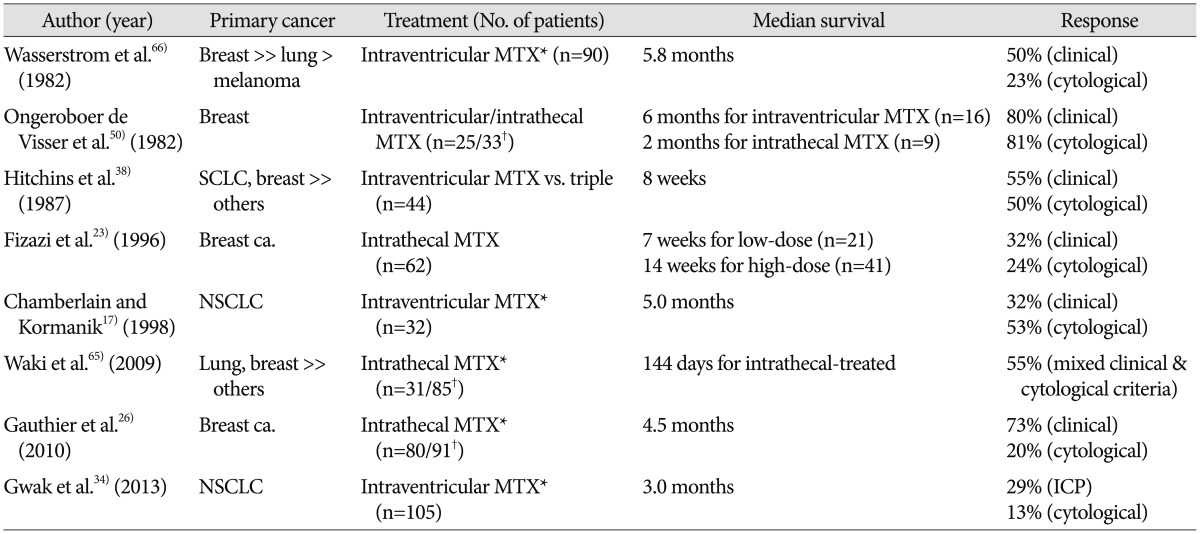
Although well-controlled, prospective or randomized study to evaluate the benefit of intra-CSF chemotherapy are lacking, intra-CSF can evidently prolong OS of patients with LMC, as compared to the untreated (Table 1)1723263438506566). However, such studies are limited by factors such as the small number of patients, heterogeneous primary cancer, different systemic cancer burdens, and various rounds of intra-CSF chemotherapy. Findings of previous studies show that 1) primary cancer of breast has a favorable prognosis as compared to lung cancer and/or melanoma, 2) more aggressive intra-CSF chemotherapy regimen/schedules show prolonged survival as a benefit of repeated treatment, and 3) increased intracranial pressure indicating a disrupted physiologic CSF flow is not only a bad prognostic factor but also a major obstacle to intra-CSF chemotherapy. Chamberlain and Kormanik17) report clinical results of 32 LMC patients from NSCLC after a relatively intense schedule of intraventricular chemotherapy, in which median survival is prolonged to 5 months. The 'intense schedule' includes the 'concentration×time' schedule administration of MTX (2 mg for 5 consecutive days every other week for 8 weeks), the second (cytosine arabinoside) and the third (Thiotepa) salvage intraventricular chemotherapy. In our study on the institutional data, median survival of 105 patients with LMC from NSCLC, who underwent a median 5 rounds of intraventricular chemotherapy via Ommaya reservoir, is 3.0 months34). Although the median OS is not impressive, we show the efficacy of intraventricular chemotherapy for patient survival using 'time-dependent covariate analysis' to eliminate the possibility of higher frequency chemotherapy given to patients who lived longer (the reverse-causation problem)34).
Due to the rarity of available drugs for intra-CSF chemotherapy, MTX in combination with or salvaged by cytosine arabinoside (Ara-C) with hydrocortisone or Thiotepa comprises most treatment regimens (Table 1). Although some patients with contraindications or resistance to MTX have some benefit from Ara-C or Thiotepa, it is still unclear whether MTX in combination with other drugs is superior to MTX monotherapy as the 1st line therapy3841). One randomized controlled study compares DepoCyt (sustained-release formulation of cytarabine; 50 mg every 2 weeks) to conventional intra-CSF MTX (10 mg twice a week for 4 weeks) in patients with LMC from solid tumors. Apparent prolonged OS in DepoCyt-treated patients (105 days vs. 78 days) has no statistical significance, but time-to-neurological progression is significantly delayed (58 days vs. 30 days)27).
LMC is defined as malignant cells in the CSF on cytology ex-amination. However, pathologic process usually occurs in the subpial CSF space, where cancer cells get lodged and cause neurological dysfunction and CSF flow obstruction. Hence, the low probability of floating cancer cells within a small amount of CSF results in 50-60% sensitivity of single CSF cytology despite overt LMC-related symptoms2866). Nevertheless, we obtain a sensitivity of 85-90% on repeated cytology examinations.
T1-weighted gadolinium enhancement MRI (Gd-MRI) of both brain and spine is the standard diagnostic procedure for patients with LMC to support the diagnosis and assess the extent of disease, including bulky disease293059). Although the 'evident enhancement of leptomeninges' on Gd-MRI reveal a sensitivity around 70% for diagnosis of LMC, the sensitivity may be as high as 90%. This is because it frequently contains 'suggestive findings' of enhancement of dura, ventricular ependyma with or without ventriculomegaly or nodular enhancement in subarachnoid space in patients with overt LMC-related symptoms2568). Combined with clinical features of LMC, Gd-MRI can support a diagnosis of LMC in patients with negative CSF cytology2556). Thus, in patients with known primary cancer who show suspected symptoms of LMC, Gd-MRI should be done before painful lumbar puncture, as it mimics leptomeningeal enhancement along with the infectious condition of CSF2947).
In context of the false negative CSF cytology for diagnosis, one-time negative conversion of CSF cytology after intra-CSF chemotherapy does not constitute a treatment response. Chamberlain and Kormanik17) propose complete response of CSF cytology as 'two consecutive negative results at least one week apart and sustained for at least one month' and partial response as 'from positive to suspicious' under the same conditions. However, these CSF responses are neither correlated with patients OS nor sustained for several months in patients with LMC from solid cancers, in contrast to prolonged CSF negative conversion in patients with leukemia at chemo-off period113456).
While direct injection of drugs into CSF space bypasses the BBB, the risk of arachnoiditis requires available drugs to be water-soluble. Simultaneously, to avoid acute neurotoxicity from direct absorption into brain parenchyma, drugs should have appropriate chemical properties for intra-CSF use. Blaney et al.9) summarize characteristics of an ideal new agent for intrathecal administration as follows : 1) absence of neurotoxicity following systemic administration, 2) a mechanism of action that differs from agents currently available for intrathecal use or a novel mechanism of action, and 3) solubility in a vehicle that is suitable for intrathecal administration. Agents that meet the above conditions require intensive preclinical study including in vitro cytotoxicity, pharmacokinetics and chronic dosing study in the non-human primate before clinical trials.
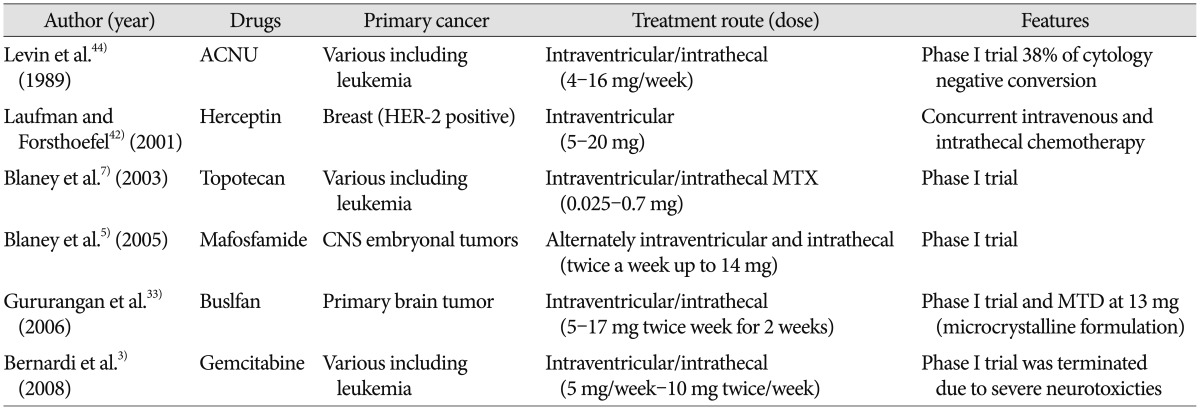
List and clinical results are summarized in Table 2.
Topotecan, a topoisomerase I inhibitor shows a wide-spectrum of anti-tumor activity. While it has a relatively high CSF penetration rate (30%), systemic or central nervous system (CNS) toxicity is rarely observed. These properties suggest the intrathecal use of topotecan, and non-human primate pharmacokinetic study show a 450-fold higher CSF concentration after an intraventricular dose of 0.1 mg than systemic administration6). Subsequently, phase I trial was performed in patients with LMC from various cancers including leukemia, breast cancer, lung cancer and gliomas7). At a maximal tolerable dose (MTD) of 0.4 mg, lumbar CSF concentration at 2 hours is 1 uM at an average 15% of ventricular concentration. Among the 23 patients ob-served, grade 3 transient arachnoiditis occurred in 3 patients and 1 patients showed grade 3 ataxia, which did not completely recover. All 3 leukemia patients and 3 of the 13 glioma patients showed CSF cytology responses but following phase II study is not yet performed.
Mafosfamide is a preactivated formulation of cylophsophamide, which shows wide spectrum anti-tumor activity but typically needs hepatic enzyme activation to become cytotoxic. As cyclophosphamide is effective for CNS embryonal tumors, which have a predilection for LMC, clinical trials are performed on young children with these tumors to prevent LMC and to delay or possibly avoid whole neuraxis radiation. Initial phase I study failed to achieve cytotoxic CSF concentration (>10 uM) due to dose-limiting toxicity (DLT) of headache and/or irritability and rapid clearance at 5 mg of mafosfamide4). However, in the following phase I study, patients could tolerate 14 mg of mafosfamide with premedication of steroid and morphine and had effective mafosfamide CSF concentration5). The recently published result of 'systemic and intrathecal mafosfamide followed by conformal radiation for infants with intracranial CNS tumor : a pediatric brain tumor consortium study (PBTC-001)' suggest that the incopration of intrathecal mafosfamide to systemic chemotherapy is feasible8).
Gemcitabine, a deoxy-citidine analog anti-metabolite, shows anti-tumor activity against various solid tumors. As gemcitabine is water-soluble but barely penetrates blood-CSF barrier after intravenous administration, it is considered as an excellent candidate for intrathecal chemotherapy. Non-human primate study of intraventricular gemcitabine administration is promising as it reveals negligible plasma gemcitabine concentration with effective CSF concentration based on pharmacokinetics22). On 5 mg per week for 4 weeks schedule, there is no discernible neurotoxicity except transient CSF pleocytosis. Thus, a phase 1 clinical trial has been launched with a basal dose of 5 mg per week (1/10th of the non-human primate dose equivalent based on the different CSF volume)3). However, DLT of 2 severe neurotoxicities (transverse myelitis and somnolence) occurred at the 10th and 7th administration of intrathecal gemcitabine without objective response other than stable disease. Phase I trial is prematurely discontinued due to different cytidine deaminase (converting gemcitabine to its inactive form) levels between non-human primates and human, lack of chronic dosing study, and prior treatment of intrathecal chemotherapy and radiation in patients show DLTs.
Non water-solubility of busulfan precludes its intrathecal administration while it shows activity against cyclophosphamide resistant neoplasms. Microcrystalline formulation (Starject bu-sulfan®, SuperGen Inc., San Ramon, CA, USA) solves the solubility problem, and successful intrathecal Starject busulfan in a nude rat model has prompted phase I clinical trial in children with primary brain tumors (PBTC-004)33). Effective CSF con-centration of 50-150 ug/mL is achieved with transient grade 3 toxicities of vomiting, headache and arachnoiditis on a 5-17 mg twice weekly for 2 weeks schedule; 13 mg of busulfan is recom-mended for future phase II study.
Levin et al.43) tested chloroethy nitrosoureas, which have ad-vantage of non-cell cycle specific agents, in a beagle model. Among the nitrosureas, water-soluble ACNU is best tolerated, and based on the observed clearance rate, the phase I baseline dose of 8 mg/week every other week is suggested44). Accumulated dose of up to 104 mg (8 mg per week×13 times) leads to no bone marrow suppression in patients. Neurotoxicities of headache, nausea, vomiting, and radiculopathy are all transient.
As monoclonal antibodies do not cross the BBB due to molecular weight, successful intrathecal Rituximab treatment in lymphoma and leukemia has prompted the introduction of monoclonal antibodies to intra-CSF chemotherapy. Among these, Hereptin (transtuzumab) is the most widely used monoclonal antibody targeting LMC from HER-2 positive breast cancer. Despite the absence of systematic phase I trial, single dose is escalated up to 150 mg but the interval is variable according to case reports39). A pooled analysis of intrathecal Herceptin reveals 2/3rd CSF response, median OS of 13.5 months and median PFS of 7.5 moths69). Other monoclonal antibodies, combined with immunotoxin or radio-isotope to increase cancer cell specificity or lethal effect are tried in animal models but not in human yet.
Recent studies report that patients with LMC from NSCLC, who receive intrathecal or intraventricular chemotherapy with concurrent RTKi, have prolonged survival compared to patients with intra-CSF chemotherapy alone345167). RTKi's partially cross the BBB and oral dose regimen of erotinib and gefitinib achieve effective CSF concentration in this low bound-protein compartment204046). Frequent clinical response in patients with parenchymal brain metastasis and/or LMC from NSCLC after RTKi, is an impetus to evaluate its efficacy in further clinical trials according to EGFR mutation.
CSF flow obstruction, found in >50% LMC patients, is a long standing major obstacle to intraventricular chemotherapy as it hinders effective distribution of injected drugs16). Most studies measuring ICP in LMC patients agree that increased ICP is one of the poor prognostic factors for patient survival183234). Compartment model of CSF flow predicts that simple diffusion cannot achieve even distribution of injected drugs in case of disturbed CSF flow, as the diffusion distance between the ventricle and lumbar subarachnoid space is too long to be reached without physiologic CSF flow10). As a consequence, increased gradient and transit time of injected drugs in the lateral ventricle facilitate penetration into brain parenchyma and causes encephalopathy3132). Siegal et al.56) report that the incidence of delayed leucoencephalopathy in LMC patients treated with intraventricular chemotherapy significantly correlates with increased ICP.
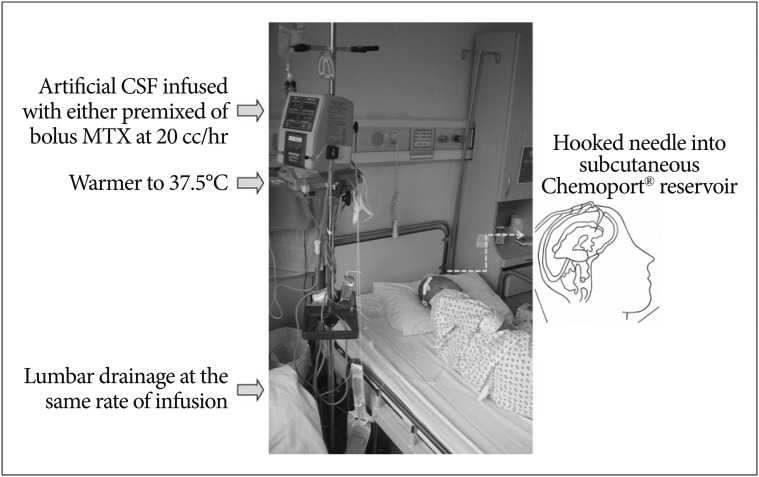
Perfusion of chemotherapeutic drug from one lateral ventricle to another or lumbar space was tried by Rubin et al.52) for the purpose of treating glioma or meningeal leukemia while reducing systemic absorption. Later, Nakagawa et al.49) attempt "ventriculolumbar perfusion" (VLP) chemotherapy to increase drug delivery to the lumbar subarchnoid space. MTX is injected into the ventricle as a bolus while artificial CSF is perfused from the ventricle to lumbar drainage for 3 days on VLP in LMC patients from solid cancers (Fig. 1). While 3 out of 6 bed-ridden patients unexpectedly achieved ambulation after VLP, all 13 patients transiently suffered from severe nausea, vomiting, headache, fever, and confusion. Nakagawa et al.49) propose further investigation to establish appropriate drug doses and perfusate volume, while they stop VLP due to the unacceptable toxicities as com-pared to standard intrathecal chemotherapy. A phase I study of VLP by Gwak et al.36) suggests both perfusion rate and daily MTX dose as variables for MTD. They introduce a continuous infusion of MTX and artificial CSF mixture to human clinical trial for the first time with proven effective CSF concentration at a lower dose of MTX than bolus injection in the non-human primate model1). Perfusion rate of 20 mL/h and daily MTX dose of 24 mg is well-tolerated in patients. The surprising control rate of increased ICP (75%) has prompted phase II trial of VLP. Subsequent phase II trial shows significantly high control rate of increased ICP (71% in 45 patients, median time-to ICP control of 104 days) and prolonged OS of 6.0 moths with 19% of improved ambulatory function including 4 bed ridden patients who became ambulatory. The study shows that the OS of NSCLC patients with LMC is double that of conventional NSCLC patients with LMC (3.0 months vs. 6.0 months, p<0.001). VLP thus shows following advantages 1) VLP can be applied to patients with increased ICP, for whom conventional intra-CSF therapy cannot be applied or is ineffective, 2) both symptom improvement rate and OS is significantly improved in these patients who have bad prognostic clinical factors. Further investigation to reduce the constitutive side effects of VLP (nausea, vomiting and confusion) by reducing perfusion rate is on-going.
As CSF cytology shows frequent false negative results, investigators have sought more predictive diagnostic values for CSF profiles such as elevated protein levels, low glucose, and increased lactate dehydrogenase/β-glucosidase/β-2 microglobulin etc. or a combination of these values37636465). However, they are consistent with, but not diagnostic of LMC, and investigators are un-able to provide cut-off values of the diagnostic variables agreeable to clinicians. Tumor specific antigens such as CA 19-9, Cyfra 21-1 or CEA have higher sensitivity and specificity than CSF cytology or other markers265364). But, they do not appear in all patients that have these primary cancers. Recent efforts to find a small amount of onco-protein, onco-DNA or onco-microRNA in CSF utilizing advances in molecular biological techniques show promising results2455860), but the pilot studies need to be validated in a large number of patients including a control group.
We also need a pharmacodynamic marker for intra-CSF chemotherapy to monitor the treatment response and to set a therapeutic end point. However, current CSF cytology examination is neither valid for complete/partial remission nor appropriate for quantitative measurement of cancer cell burden. Quantitative measurement of cancer metabolites or exosomes is on-going, but a correlation with clinical status is not yet proven4562). Subpial deposit of cancer cells is more commonly found than free floating cells in the CSF flow, hence simple measurement of CSF values does not fully represent the cancer cell burden of LMC. Thus, elaborate analyses of these variables should combine clinical information such as the post-treatment time of CSF sample and the patient's clinical status.
Prognosis of patients with LMC from solid tumors is still dismal without a cure despite intra-CSF chemotherapy. However, major problems of intra-CSF chemotherapy can be solved by developing more available drugs to overcome drug-resistance and advancement of drug delivering technique such as VLP. Elaborate search for biomarker/pharmacodynamic marker combined with clinical findings would greatly facilitate the introduction of new drugs and technologies to clinical trials for LMC.
References
1. Balis FM, Blaney SM, McCully CL, Bacher JD, Murphy RF, Poplack DG. Methotrexate distribution within the subarachnoid space after intraventricular and intravenous administration. Cancer Chemother Pharmacol. 2000; 45:259–264. PMID: 10663645.

2. Baraniskin A, Kuhnhenn J, Schlegel U, Schmiegel W, Hahn S, Schroers R. MicroRNAs in cerebrospinal fluid as biomarker for disease course monitoring in primary central nervous system lymphoma. J Neurooncol. 2012; 109:239–244. PMID: 22729947.

3. Bernardi RJ, Bomgaars L, Fox E, Balis FM, Egorin MJ, Lagattuta TF, et al. Phase I clinical trial of intrathecal gemcitabine in patients with neoplastic meningitis. Cancer Chemother Pharmacol. 2008; 62:355–361. PMID: 17909804.

4. Blaney SM, Balis FM, Berg S, Arndt CA, Heideman R, Geyer JR, et al. Intrathecal mafosfamide : a preclinical pharmacology and phase I trial. J Clin Oncol. 2005; 23:1555–1563. PMID: 15735131.
5. Blaney SM, Boyett J, Friedman H, Gajjar A, Geyer R, Horowtiz M, et al. Phase I clinical trial of mafosfamide in infants and children aged 3 years or younger with newly diagnosed embryonal tumors : a pediatric brain tumor consortium study (PBTC-001). J Clin Oncol. 2005; 23:525–531. PMID: 15659498.

6. Blaney SM, Cole DE, Balis FM, Godwin K, Poplack DG. Plasma and cerebrospinal fluid pharmacokinetic study of topotecan in nonhuman primates. Cancer Res. 1993; 53:725–727. PMID: 8428353.
7. Blaney SM, Heideman R, Berg S, Adamson P, Gillespie A, Geyer JR, et al. Phase I clinical trial of intrathecal topotecan in patients with neoplastic meningitis. J Clin Oncol. 2003; 21:143–147. PMID: 12506183.

8. Blaney SM, Kocak M, Gajjar A, Chintagumpala M, Merchant T, Kieran M, et al. Pilot study of systemic and intrathecal mafosfamide followed by conformal radiation for infants with intracranial central nervous system tumors : a pediatric brain tumor consortium study (PBTC-001). J Neurooncol. 2012; 109:565–571. PMID: 22790443.

9. Blaney SM, Poplack DG. New cytotoxic drugs for intrathecal administration. J Neurooncol. 1998; 38:219–223. PMID: 9696375.
10. Blasberg RG, Patlak CS, Shapiro WR. Distribution of methotrexate in the cerebrospinal fluid and brain after intraventricular administration. Cancer Treat Rep. 1977; 61:633–641. PMID: 406996.
11. Bokstein F, Lossos A, Lossos IS, Siegal T. Central nervous system relapse of systemic non-Hodgkin’s lymphoma : results of treatment based on high-dose methotrexate combination chemotherapy. Leuk Lymphoma. 2002; 43:587–593. PMID: 12002763.

12. Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors : a comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998; 82:1756–1763. PMID: 9576299.
13. Boogerd W, van den Ben MJ, Koehler PJ, Heimans JJ, van der Sande JJ, Aaronson NK, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer : a randomised study. Eur J Cancer. 2004; 40:2726–2733. PMID: 15571954.

14. Bruna J, González L, Miró J, Velasco R, Gil M, Tortosa A, et al. Leptomeningeal carcinomatosis : prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009; 115:381–389. PMID: 19109820.
15. Chamberlain MC. Leptomeningeal metastases : a review of evaluation and treatment. J Neurooncol. 1998; 37:271–284. PMID: 9524085.
16. Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998; 38:135–140. PMID: 9696363.
17. Chamberlain MC, Kormanik P. Carcinoma meningitis secondary to non-small cell lung cancer : combined modality therapy. Arch Neurol. 1998; 55:506–512. PMID: 9561978.

18. Chamberlain MC, Kormanik PA. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996; 46:1674–1677. PMID: 8649568.

19. Chamberlain MC, Kormanik PA, Barba D. Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg. 1997; 87:694–699. PMID: 9347977.

20. Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010; 99:283–286. PMID: 20146086.

21. de Azevedo CR, Cruz MR, Chinen LT, Peres SV, Peterlevitz MA, de Azevedo Pereira AE, et al. Meningeal carcinomatosis in breast cancer : prognostic factors and outcome. J Neurooncol. 2011; 104:565–572. PMID: 21234642.

22. Egorin MJ, Zuhowski EG, McCully CM, Blaney SM, Kerr JZ, Berg SL, et al. Pharmacokinetics of intrathecal gemcitabine in nonhuman primates. Clin Cancer Res. 2002; 8:2437–2442. PMID: 12114450.
23. Fizazi K, Asselain B, Vincent-Salomon A, Jouve M, Dieras V, Palangie T, et al. Meningeal carcinomatosis in patients with breast carcinoma. Clinical features, prognostic factors, and results of a high-dose intrathecal methotrexate regimen. Cancer. 1996; 77:1315–1323. PMID: 8608509.

24. Fleischhack G, Jaehde U, Bode U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin Pharmacokinet. 2005; 44:1–31. PMID: 15634030.

25. Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995; 38:51–57. PMID: 7611725.

26. Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010; 21:2183–2187. PMID: 20430906.

27. Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999; 5:3394–3402. PMID: 10589750.
28. Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979; 29:1369–1375. PMID: 573381.

29. Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006; 5:443–452. PMID: 16632315.

30. Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999; 25:103–119. PMID: 10395835.

31. Grossman SA, Reinhard CS, Loats HL. The intracerebral penetration of intraventricularly administered methotrexate : a quantitative autoradiographic study. J Neurooncol. 1989; 7:319–328. PMID: 2585028.

32. Grossman SA, Trump DL, Chen DC, Thompson G, Camargo EE. Cerebrospinal fluid flow abnormalities in patients with neoplastic meningitis. An evaluation using 111indium-DTPA ventriculography. Am J Med. 1982; 73:641–647. PMID: 6814249.

33. Gururangan S, Petros WP, Poussaint TY, Hancock ML, Phillips PC, Friedman HS, et al. Phase I trial of intrathecal spartaject busulfan in children with neoplastic meningitis : a Pediatric Brain Tumor Consortium Study (PBTC-004). Clin Cancer Res. 2006; 12:1540–1546. PMID: 16533779.

34. Gwak HS, Joo J, Kim S, Yoo H, Shin SH, Han JY, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol. 2013; 8:599–605. PMID: 23422833.

35. Gwak HS, Joo J, Shin SH, Yoo H, Han JY, Kim HT, et al. Ventriculolumbar perfusion chemotherapy with methotrexate for treating leptomeningeal carcinomatosis : a Phase II Study. Oncologist. 2014; 19:1044–1045. PMID: 25209375.

36. Gwak HS, Lim HS, Shin SH, Yoo H, Han JY, Kim HT, et al. Ventriculolumbar perfusion chemotherapy for the treatment of leptomeningeal carcinomatosis : a phase I study with pharmacokinetic data. Am J Clin Oncol. 2013; 36:491–499. PMID: 22781384.

37. Herrlinger U, Förschler H, Küker W, Meyermann R, Bamberg M, Dichgans J, et al. Leptomeningeal metastasis : survival and prognostic factors in 155 patients. J Neurol Sci. 2004; 223:167–178. PMID: 15337619.

38. Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987; 5:1655–1662. PMID: 3309199.

39. Hofer S, Mengele K, Stemmler HJ, Schmitt M, Pestalozzi B. Intrathecal trastuzumab : dose matters. Acta Oncol. 2012; 51:955–956. PMID: 22524214.
40. Katayama T, Shimizu J, Suda K, Onozato R, Fukui T, Ito S, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009; 4:1415–1419. PMID: 19692934.

41. Kim DY, Lee KW, Yun T, Park SR, Jung JY, Kim DW, et al. Comparison of intrathecal chemotherapy for leptomeningeal carcinomatosis of a solid tumor : methotrexate alone versus methotrexate in combination with cytosine arabinoside and hydrocortisone. Jpn J Clin Oncol. 2003; 33:608–612. PMID: 14769837.

42. Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clin Breast Cancer. 2001; 2:235. PMID: 11899418.

43. Levin VA, Byrd D, Campbell J, Giannini DD, Borcich JK, Davis RL. Central nervous system toxicity and cerebrospinal fluid pharmacokinetics of intraventricular 3-[(4-amino-2-methyl-5-pyrimidinyl)ethyl]-1-(2-chloroethyl)-1-nitro soureas and other nitrosoureas in beagles. Cancer Res. 1985; 45:3803–3809. PMID: 3860287.
44. Levin VA, Chamberlain M, Silver P, Rodriguez L, Prados M. Phase I/II study of intraventricular and intrathecal ACNU for leptomeningeal neoplasia. Cancer Chemother Pharmacol. 1989; 23:301–307. PMID: 2706735.

45. Liu S, Sun J, Lan Q. Glioblastoma microvesicles promote endothelial cell proliferation through Akt/beta-catenin pathway. Int J Clin Exp Pathol. 2014; 7:4857–4866. PMID: 25197356.
46. Masuda T, Hattori N, Hamada A, Iwamoto H, Ohshimo S, Kanehara M, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol. 2011; 67:1465–1469. PMID: 21274533.

47. Mittl RL Jr, Yousem DM. Frequency of unexplained meningeal enhancement in the brain after lumbar puncture. AJNR Am J Neuroradiol. 1994; 15:633–638. PMID: 8010262.
48. Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, et al. Leptomeningeal metastasis from non-small cell lung cancer : survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012; 7:382–385. PMID: 22089116.

49. Nakagawa H, Fujita T, Kubo S, Izumoto S, Nakajima Y, Tsuruzono K, et al. Ventriculolumbar perfusion chemotherapy with methotrexate and cytosine arabinoside for meningeal carcinomatosis : a pilot study in 13 patients. Surg Neurol. 1996; 45:256–264. PMID: 8638223.
50. Ongerboer de Visser BW, Somers R, Nooyen WH, van Heerde P, Hart AA, McVie JG. Intraventricular methotrexate therapy of leptomeningeal metastasis from breast carcinoma. Neurology. 1983; 33:1565–1572. PMID: 6685829.

51. Park JH, Kim YJ, Lee JO, Lee KW, Kim JH, Bang SM, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012; 76:387–392. PMID: 22186628.

52. Rubin RC, Ommaya AK, Henderson ES, Bering EA, Rall DP. Cerebrospinal fluid perfusion for central nervous system neoplasms. Neurology. 1966; 16:680–692. PMID: 5949435.

53. Sato Y, Ohta Y, Kaji M, Oizumi K, Kaji M. Carbohydrate antigen 19-9 in cerebrospinal fluid and within malignant cells in a case of leptomeningeal carcinomatosis. J Neurol Neurosurg Psychiatry. 1998; 65:402–403. PMID: 9728963.

54. Schabet M, Kloeter I, Adam T, Heidemann E, Wiethölter H. Diagnosis and treatment of meningeal carcinomatosis in ten patients with breast cancer. Eur Neurol. 1986; 25:403–411. PMID: 3792399.

55. Shapiro WR, Young DF, Mehta BM. Methotrexate : distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975; 293:161–166. PMID: 806016.

56. Siegal T, Lossos A, Pfeffer MR. Leptomeningeal metastases : analysis of 31 patients with sustained off-therapy response following combined-modality therapy. Neurology. 1994; 44:1463–1469. PMID: 8058150.
57. Straathof CS, de Bruin HG, Dippel DW, Vecht CJ. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999; 246:810–814. PMID: 10525979.

58. Swinkels DW, de Kok JB, Hanselaar A, Lamers K, Boerman RH. Early detection of leptomeningeal metastasis by PCR examination of tumor-derived K-ras DNA in cerebrospinal fluid. Clin Chem. 2000; 46:132–133. PMID: 10620587.

59. Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy : a review. J Neurooncol. 2005; 75:85–99. PMID: 16215819.

60. Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012; 14:689–700. PMID: 22492962.

61. Tetef ML, Margolin KA, Doroshow JH, Akman S, Leong LA, Morgan RJ Jr, et al. Pharmacokinetics and toxicity of high-dose intravenous methotrexate in the treatment of leptomeningeal carcinomatosis. Cancer Chemother Pharmacol. 2000; 46:19–26. PMID: 10912573.

62. Tran HC, Gardner S, Weiner HL, Liebes LF, Finlay JL. Pilot study assessing a seven-day continuous intrathecal topotecan infusion for recurrent or progressive leptomeningeal metastatic cancer. J Oncol Pharm Pract. 2014; 20:229–232. PMID: 23929729.

63. Twijnstra A, van Zanten AP, Hart AA, Ongerboer de Visser BW. Serial lumbar and ventricle cerebrospinal fluid lactate dehydrogenase activities in patients with leptomeningeal metastases from solid and haematological tumours. J Neurol Neurosurg Psychiatry. 1987; 50:313–320. PMID: 3559613.

64. Twijnstra A, van Zanten AP, Nooyen WJ, Ongerboer de Visser BW. Sensitivity and specificity of single and combined tumour markers in the diagnosis of leptomeningeal metastasis from breast cancer. J Neurol Neurosurg Psychiatry. 1986; 49:1246–1250. PMID: 3540216.

65. Waki F, Ando M, Takashima A, Yonemori K, Nokihara H, Miyake M, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009; 93:205–212. PMID: 19043775.

66. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors : experience with 90 patients. Cancer. 1982; 49:759–772. PMID: 6895713.

67. Yi HG, Kim HJ, Kim YJ, Han SW, Oh DY, Lee SH, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009; 65:80–84. PMID: 19059670.

68. Yousem DM, Patrone PM, Grossman RI. Leptomeningeal metastases : MR evaluation. J Comput Assist Tomogr. 1990; 14:255–261. PMID: 2312855.
69. Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer : a systematic review and pooled analysis. Breast Cancer Res Treat. 2013; 139:13–22. PMID: 23588955.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download