Abstract
Objective
The purpose of this study was to investigate changes in the plasma level of vascular endothelial growth factor (VEGF) after Gamma Knife radiosurgery (GKRS) for the treatment of meningioma.
Methods
Fourteen patients with meningiomas had peripheral venous blood collected at the time of GKRS and at 1 week, 1 month, 3 month and 6 month visits. Plasma VEGF levels were measured using commercially available enzyme-linked immunosorbent assay. For controls, peripheral blood samples were obtained from 20 healthy volunteers.
Results
The mean plasma VEGF level (29.6 pg/mL) in patients with meningiomas before GKRS was significantly lower than that of the control group (62.4 pg/mL, p=0.019). At 1 week after GKRS, the mean plasma VEGF levels decreased to 23.4 pg/mL, and dropped to 13.9 pg/mL at 1 month, 14.8 pg/mL at 3 months, then increased to 27.7 pg/mL at 6 months. Two patients (14.3%) with peritumoral edema (PTE) showed a level of VEGF 6 months after GKRS higher than their preradiosurgical level. There was no significant association found in an analysis of correlation between PTE and tumor size, marginal dose, age, and sex.
Recently, stereotactic radiosurgery (SRS) for meningiomas has been used in cases that are inoperable, or when the tumor has recurred or was not completely removed. Even though the control rates for the SRS are high, a qualitative analysis of treatment outcome after Gamma Knife radiosurgery (GKRS) may be needed to understand the effectiveness and complications of GKRS1,8,16).
Angiogenesis plays an important role in the development and progression of intracranial tumors, especially in meningioma3). This angiogenesis is primarily mediated through vascular endothelial growth factor (VEGF). With stimulation by hypoxia, VEGF involves in the formation of new blood vessels. Based on its potential to increase vascular permeability, Senger et al.15) first hypothesized an involvement of VEGF in angiogenesis. It is also indirectly involved in the development of peritumoral edema (PTE). PTE has been widely reported subsequent to GKRS. Edema developed in 25% to 71% of patients with nonbasal menigniomas, compared with 0 to 22% of basal meningiomas2,7,10,17). The direct cause of PTE is still not known although many factors have been suggested including tumor location, size, and radiation dose1,2,4,10,11,14).
It is known that meningiomas produce vasogenic mediators and high levels of VEGF concentration at the tissue level; however, evaluation of plasma VEGF in patients with meningioma before and after SRS has not yet been demonstrated. VEGF found in the blood may aid in understanding the pathophysiology of tumor response and radiation-induced complications.
In this study, we evaluated plasma VEGF of patients with meningioma before and after GKRS, and investigated the changes in this biomarker to predict treatment efficacy and radiation-induced complications after GKRS.
Fourteen consecutive patients with meningioma, all of whom provided written informed consent, were enrolled in a prospective protocol that was reviewed and approved by the institutional review board at our hospital. Thirteen of these patients underwent GKRS as the primary treatment for meningiomas, and the remaining one patient underwent GKRS following surgical resection. Peripheral blood samples donated from 20 volunteers without known malignancy or pregnancy were used as controls.
Peripheral venous whole blood was obtained before radiosurgery and 1 week, 1 month, 3 months, and 6 months after GKRS. Blood samples from protocol participants were collected in citrate-supplemented tubes and centrifuged at 3200 rpm for 10 minutes at 4℃. The supernatant including plasma fraction was transferred into microtube and immediately frozen in aliquots at -80℃ until analysis. The analysis was performed with a commercially available ELISA kit (Quantikine Human VEGF Immunoassay, all from R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. All samples collected from a patient were assayed simultaneously.
SRS was carried out using a Gamma Knife Model C (Elekta AB, Stockholm, Sweden). T1-, T2-, and enhanced T1-weighted magnetic resonance (MR) images with a slice thickness of 2 mm were used for the 3-dimensional reconstructions and treatment planning. The MR images were transferred to a workstation for post-processing and analysis. The Gamma Plan system was used to determine the GKRS for all patients. To deliver a highly conformal dose to the tumor, multiple small isocenters were used. The volume of the tumor ranged from 0.8 cc to 14.6 cc (median, 5.0 cc). The median marginal dose was 12.5 Gy (8-14 Gy). In all patients, the 50% isodose line was used for the margin. The maximal radiation doses varied between 16 and 28 Gy (median, 25.0 Gy). The size of each tumor and PTE, before and 6 months after GKRS, was retrospectively measured using the Gamma Plan workstation and picture archiving and communicating system system.
All analyses were performed using SPSS 18.0 for Windows (IBM Corporation, Chicago, IL, USA). Because the data were not distributed normally, they were analyzed using the Wilcoxon rank sum test, the Mann-Whitney U test, and the Kruskal-Wallis test. Correlations were assessed using the nonparametric Spearman rank correlation. p values less than 0.05 were accepted as the threshold for statistical significance.
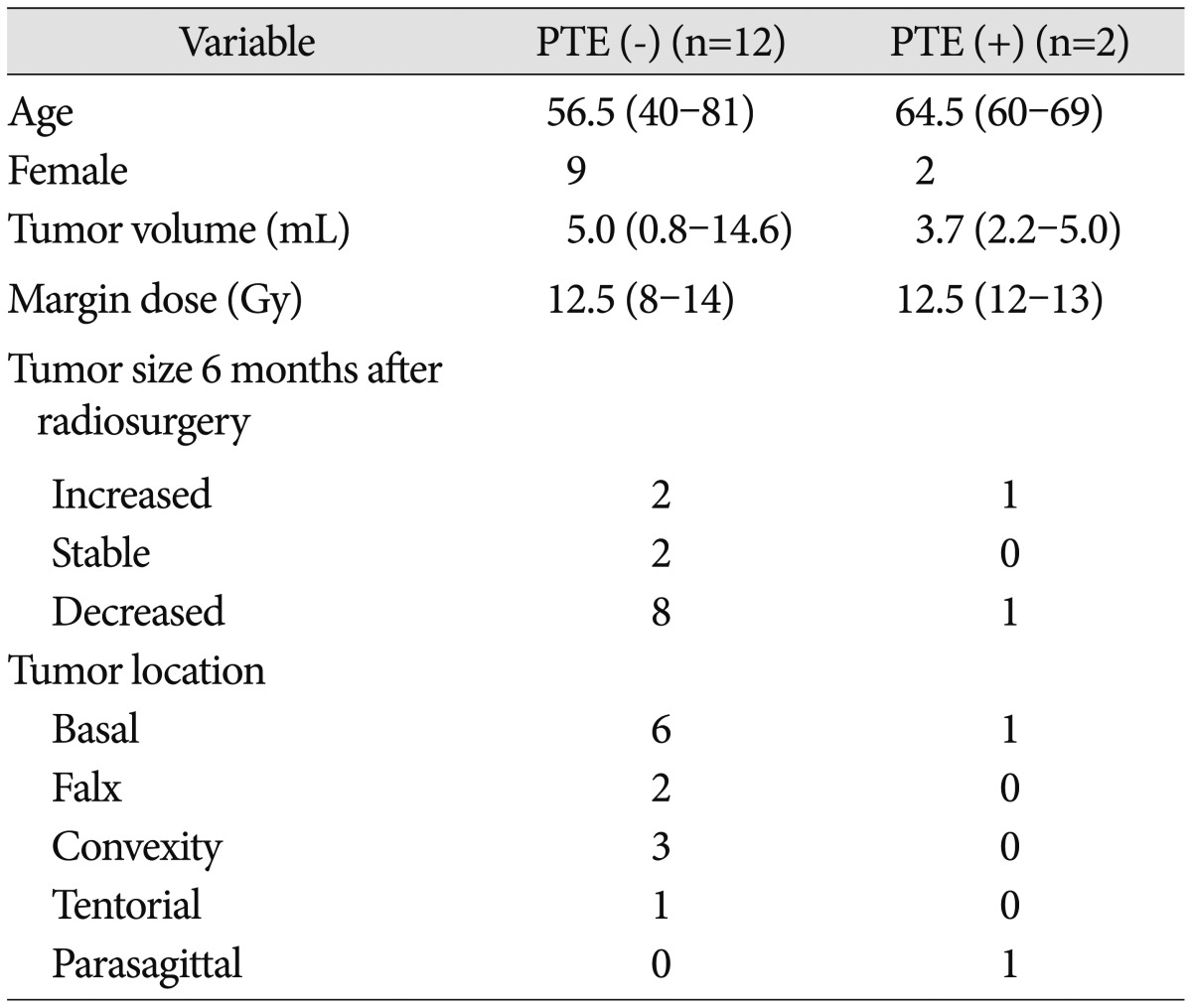
Of the 14 consecutive patients enrolled, 11 were women and 3 were men (median age 59 years, range 40-81). In the 20 controls, there were 5 women and 15 men and their median age was 49.5 years (range 30-63). Seven patients had basal meningiomas (50%), 3 had meningiomas located on the convexity (21.4%), 2 had falx meningiomas (14.3%), 1 had tentorial meningioma (7.1%), and 1 had parasagittal meningioma (7.1%). The patient and tumor characteristics, along with radiosurgical parameters, are summarized in Table 1. At the 6 months MR imaging, 64.3% of tumors decreased in size, 14.3% were stable, and 21.4% increased in size.
The mean plasma VEGF level in patients with meningioma was 29.6 pg/mL (range 0.59-106.2) before GKRS, and 62.4 pg/mL (range 9.2-153.1) in the control group. VEGF levels in patients with meningiomas were significantly lower than that of the control group (p=0.019). In the control group, there was no significant difference in VEGF levels between the sexes (p=0.274). VEGF levels were not significantly correlated with age (p=0.103). In patients, there was no significant difference in VEGF levels between the sexes (p=0.160). No correlation was found between plasma VEGF level and patient age (p=0.403) or tumor size (p=0.075).
At 1 week after GKRS, the mean plasma VEGF levels decreased to 23.4 pg/mL (median, 18.2 pg/mL), then dropped to 13.9 pg/mL (median, 11.6 pg/mL) at 1 month, 14.8 pg/mL at 3 months (median, 5.7 pg/mL), and finally increased to 27.7 pg/mL (median, 20.3 pg/mL) at 6 months (Fig. 1). Four of 14 patients experienced a constant decrease of VEGF concentration. However, 8 patients showed a temporary increase within 3 months after GKRS, with a slow decline thereafter. The mean plasma VEGF levels in patients with an increase in tumor volume at 6 months MRI were 32.2 pg/mL before GKRS, 25.7 pg/mL at 1 week, 16.5 pg/mL at 1 month, 17.7 pg/mL at 3 months, and 27.7 pg/mL at 6 months (Table 2). The mean plasma VEGF levels in patients with a decrease and no change in tumor size were 20.3 pg/mL before GKRS, 14.8 pg/mL at 1 week, 4.4 pg/mL at 1 month, 4.4 pg/mL at 3 months, and 37.9 pg/mL at 6 months. There was no significant difference in plasma VEGF levels between the variable tumor responses at the different time following GKRS (p=0.769, 0.368, 0.088, 0.368, and 0.857, respectively).
Two patients (14.3%) experienced PTE after GKRS (Fig. 2). The plasma VEGF levels 6 months after GKRS in patients with PTE increased to 37.9 pg/mL, 42.4 pg/mL, respectively. None of the two patients had preradiosurgerical PTE. One patient had a sphenoid ridge meningioma and the other had a parasagittal meningioma involving superior sagittal sinus. Age, sex, radiation dose, and tumor size were not significantly correlated with development of PTE (p=0.410, 0.604, 0.849, and 0.855, respectively) (Table 3). The average VEGF levels for non-PTE patients decreased 16.0 pg/mL from baseline to 6 months; on the other hand, the average VEGF levels for PTE patients increased 23.3 pg/mL over the same period. However, the difference the two groups was on the borderline for statistical significance (p=0.053).
Angiogenesis is important for the development and progression of brain tumors and has been a recent therapeutic target. VEGF is the most potent angiogenic factor known so far9,12,18). It stimulates endothelial cell proliferation and enhances vascular permeability. Among many types of brain tumors, the highest levels of VEGF concentrations were detected in plasma derived from patients suffering from meningioma3). For the treatment of meningioma, surgical resection remains the best options. SRS has become a well-accepted alterative modality; however, the most frequent side effect after GKRS is the occurrence of PTE, which can cause neurological deficits in patient16).
In the present study, we evaluated plasma VEGF levels in a healthy control group and in patients with meningioma before and after GKRS in order to understand the complex mechanism of angiogenesis in tumor growth and treatment. Our finding from serial measurement of plasma VEGF suggests that VEGF may play a role in monitoring tumor response and radiation-induced complications following SRS for meningioma.
Plasma VEGF was detectable in the peripheral blood of normal healthy volunteers in a previous report18). The origin and biologic role of VEGF in controls are unknown; however, their finding implies a role for endothelial mitogens in the maintenance of physiologic endothelial integrity9). Unexpectedly, plasma VEGF concentration in controls was significantly higher than that in patients with meningiomas in our study. Our finding contrasts with studies for systemic cancer and controls6,12). However, in a recent study, plasma VEGF levels at baseline in patients harboring arteriovenous malformations were significantly decreased when compared to their control population5). Kim et al.5) suggests that elevated local VEGF production may cause the release of a molecular mediator which activates an inhibitory feedback mechanism that downregulates systemic VEGF production. However, this hypothesis is not sufficient to justify the perplexing findings of lower plasma VEGF level in patients with meningiomas. We need to collect much more data in order to understand normal or abnormal plasma VEGF levels in patients with meningiomas.
After meningiomas were treated by SRS, the plasma VEGF levels decreased 1 week, 1 month, and 3 months, consecutively; theses levels returned to their baseline values after 6 months. This return to baseline levels implies that counter-regulatory mechanisms are activated by modulation of angiogenic balance after GKRS. Meanwhile, following surgical resection of arteriovenous malformation and renal cell carcinoma, it took 5 days-1 month to return to baseline levels of VEGF5,6). This suggests that activation of counter-regulatory mechanisms after SRS for meningioma takes longer in comparison with surgery. Eight (57.1%) patients showed a temporary increase within 3 months after GKRS. The transient increase may be thought to be related to the induction of tissue hypoxia following radiosurgery, which leads to VEGF production.
In the current study, there were no significant differences in plasma VEGF levels between the patients with an increase in tumor volume and a decrease or no change 6 months after GKRS. The meningioma response following SRS occurs slowly and progressively8). Tumor regression may take up to several years following radiosurgery. Our preliminary experience could not be enough to explain the relation between tumor control and plasma VEGF levels. Meanwhile, Preusser et al.13) suggested that microvessel density (MVD) value was generally higher in meningiomas with expression of VEGF. High MVD correlated with unfavorable prognosis in their series of recurring meningiomas. VEGF seems important for tumor growth and recurrence in meningiomas. VEGF may be used to identify patients at risk of tumor recurrence as well as PTE.
VEGF is one of the molecular markers of hypoxia/hypoxia regulated angiogenesis and is increased in the area of necrosis in tumors. Twelve of 14 patients experienced a decreased pattern of VEGF after radiosurgery. However, 2 patients with PTE had higher levels of VEGF 6 months after GKRS as compared to the preradiosurgical concentration. VEGF is crucial in vascular permeability as well as angiogenesis and therefore indirectly in PTE formation in menigiomas14). Kan et al.4) suggested that increased vascular permeability induced by VEGF predisposes meningiomas to vasogenic PTE after SRS. Our results suggest an association between the development of PTE and high VEGF. Due to the small sample size and a result which is on the borderline of statistical significance, more investigation into this possible association is necessary.
The major roles of VEGF in meningioma could be the induction of neovascularization for tumor growth and the development of vasogenic edema. VEGF is a potential target for clinical research involving patients with meningioma. Anti-VEGF agents are under investigation for the treatment of meningioma and PTE after radiosurgery. Plasma VEGF measurement may be a useful tool to monitor the efficacy of anti-VEGF agents for treating not only VEGF-secreting tumor cells, but also PTE.
There are some limitations of the present study. This study had a small number of patients and a relatively short follow-up period. As a result the statistics were flawed by the sample size. The hypothesis in this study was that plasma VEFG levels might change in response to SRS for meningiomas, however, the control group had higher basal levels compared to patients with menigiomas. We need sufficient data related to plasma VEGF levels in control group and abnormal levels in brain tumor patients to use plasma VEGF levels as a potential diagnostic tool for tumor response after SRS. Plasma VEGF levels may be different according to tumor vascularity, tumor location, PTE, and the timing of the sampling. A controlled systematic prospective study with a longer follow up is necessary in order to evaluate the treatment outcome of meningioma following GKRS.
Measurement of VEGF in the peripheral blood is a relatively simple and reproducible way for monitoring the activity of angiogenesis and vascular permeability. Our study is an early data study of the potential association of plasma VEGF levels and meningiomas. It may support evidence for further work related to whether plasma VEGF levels become a useful tool for tumor response after SRS.
Acknowledgements
We thank Wade Martin for his editorial assistance in preparing this manuscript.
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2012).
References
1. Chang JH, Chang JW, Choi JY, Park YG, Chung SS. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003; 74:226–230. PMID: 12531956.

2. Ganz JC, Schröttner O, Pendl G. Radiation-induced edema after Gamma Knife treatment for meningiomas. Stereotact Funct Neurosurg. 1996; 66(Suppl 1):129–133. PMID: 9032853.

3. Ilhan A, Gartner W, Neziri D, Czech T, Base W, Hörl WH, et al. Angiogenic factors in plasma of brain tumour patients. Anticancer Res. 2009; 29:731–736. PMID: 19331229.
4. Kan P, Liu JK, Wendland MM, Shrieve D, Jensen RL. Peritumoral edema after stereotactic radiosurgery for intracranial meningiomas and molecular factors that predict its development. J Neurooncol. 2007; 83:33–38. PMID: 17245625.

5. Kim GH, Hahn DK, Kellner CP, Hickman ZL, Komotar RJ, Starke RM, et al. Plasma levels of vascular endothelial growth factor after treatment for cerebral arteriovenous malformations. Stroke. 2008; 39:2274–2279. PMID: 18535271.

6. Klatte T, Böhm M, Nelius T, Filleur S, Reiher F, Allhoff EP. Evaluation of peri-operative peripheral and renal venous levels of pro- and anti-angiogenic factors and their relevance in patients with renal cell carcinoma. BJU Int. 2007; 100:209–214. PMID: 17428240.

7. Kondziolka D, Flickinger JC, Perez B. Gamma Knife Meningioma Study Group. Judicious resection and/or radiosurgery for parasagittal meningiomas : outcomes from a multicenter review. Neurosurgery. 1998; 43:405–413. discussion 413-414. PMID: 9733295.

8. Kondziolka D, Levy EI, Niranjan A, Flickinger JC, Lunsford LD. Long-term outcomes after meningioma radiosurgery : physician and patient perspectives. J Neurosurg. 1999; 91:44–50. PMID: 10389879.

9. Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999; 85:178–187. PMID: 9921991.

10. Nakamura S, Hiyama H, Arai K, Nakaya K, Sato H, Hayashi M, et al. Gamma Knife radiosurgery for meningiomas : four cases of radiation-induced edema. Stereotact Funct Neurosurg. 1996; 66(Suppl 1):142–145. PMID: 9032855.

11. Novotný J Jr, Kollová A, Liscák R. Prediction of intracranial edema after radiosurgery of meningiomas. J Neurosurg. 2006; 105(Suppl):120–126. PMID: 18503344.

12. Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma : a prospective study. Ann Surg. 2001; 233:227–235. PMID: 11176129.

13. Preusser M, Hassler M, Birner P, Rudas M, Acker T, Plate KH, et al. Microvascularization and expression of VEGF and its receptors in recurring meningiomas : pathobiological data in favor of anti-angiogenic therapy approaches. Clin Neuropathol. 2012; 31:352–360. PMID: 22541785.

14. Schmid S, Aboul-Enein F, Pfisterer W, Birkner T, Stadek C, Knosp E. Vascular endothelial growth factor : the major factor for tumor neovascularization and edema formation in meningioma patients. Neurosurgery. 2010; 67:1703–1708. discussion 1708. PMID: 21107201.

15. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219:983–985. PMID: 6823562.

16. Torres RC, Frighetto L, De Salles AA, Goss B, Medin P, Solberg T, et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003; 14:e5. PMID: 15669816.

17. Vermeulen S, Young R, Li F, Meier R, Raisis J, Klein S, et al. A comparison of single fraction radiosurgery tumor control and toxicity in the treatment of basal and nonbasal meningiomas. Stereotact Funct Neurosurg. 1999; 72(Suppl 1):60–66. PMID: 10681692.

18. Yamamoto Y, Toi M, Kondo S, Matsumoto T, Suzuki H, Kitamura M, et al. Concentrations of vascular endothelial growth factor in the sera of normal controls and cancer patients. Clin Cancer Res. 1996; 2:821–826. PMID: 9816236.
Fig. 1
Plasma concentrations of VEGF before and after Gamma Knife radiosurgery (GKRS). VEGF : vascular endothelial growth factor.
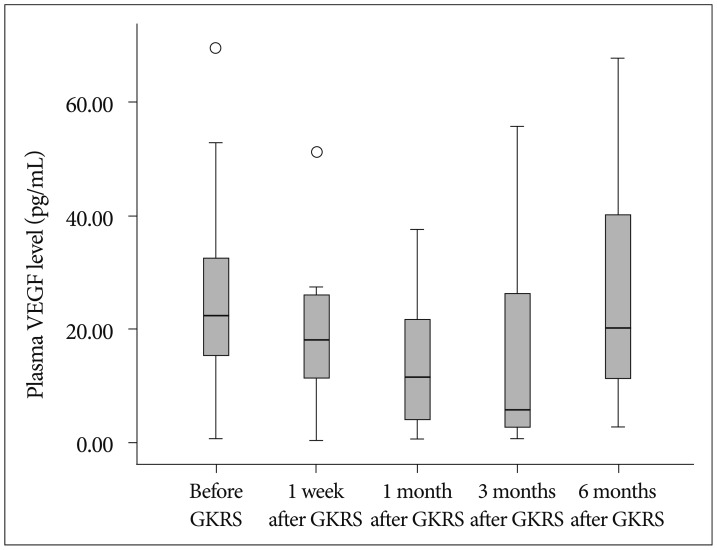
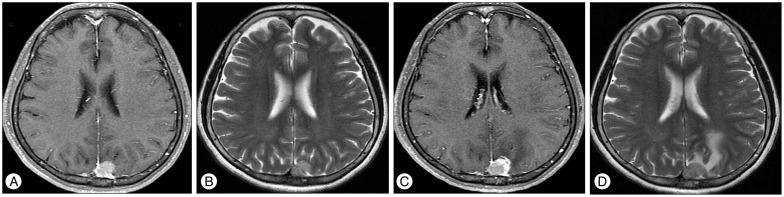
Fig. 2
Enhanced T1-weighted (A) and T2-weighted (B) magnetic resonance (MR) imaging obtained in a 60-year-old women who had a parasagittal meningioma. GKRS was performed with a margin dose of 13 Gy to the 50% isodose line. The MR image obtained 6 months after GKRS shows increased tumor volume from 1.6 mm3 to 2.2 mm3 (C) and a new peritumoral edema (D). GKRS : Gamma Knife radiosurgery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download