Abstract
Objective
Tumor necrosis factor alpha (TNF-α) have proven effects in pathogenesis of neuroinflammation after spinal cord injury (SCI). Current study is designed to evaluate the effects of an anti-TNF-α agent, adalimumab, on spinal cord clip compression injury in rats.
Methods
Thirty two male adult Wistar rats were divided into four groups (sham, trauma, infliximab, and adalimumab groups) and SCI was introduced using an aneurysm clip. Animals in treatment groups received 5 mg/kg subcutaneous adalimumab and infliximab right after the trauma. Malondialdehyde (MDA) levels were studied in traumatized spinal cord tissues 72 hours after the injury as a marker of lipid peroxidation.
Results
Animals that received anti-TNF-α agents are found to have significantly decreased MDA levels. MDA levels were significantly different between the trauma and infliximab groups (p<0.01) and trauma and adalimumab groups (p=0.022). There was no significant difference in neurological evaluation of the rats using Tarlov scale.
Spinal cord injury (SCI) with its devastating burden both for the victim and the health care system continues to be a great problem without significant improvement in management. The annual incidence of SCI is estimated as 10-50 cases per million37,40) and there is no established treatment modality on clinical base except for methylprednisolone even though the effect is controversial7,8,9,10,11,12).
Present basic science experiment is designed to demonstrate the effects of a potential pharmacological agent-adalimumab (Humira, Abbott, Abbott Park, IL, USA)-on SCI in rats. Previously we have demonstrated the effects of a tumor necrosis factor-alpha (TNF-α) inhibitor infliximab's (Remicade, Merck&Co, Whitehouse Station, NJ, USA) effects on different experimental models of neuronal injury24,31,32). Adalimumab, human recombinant monoclonal IgG1 antibody specific for cytokine TNF-α, is the third TNF-α inhibitor approved for inflammatory diseases such as ulcerative colitis after infliximab and etanercept14).
As emerged from the experimental knowledge, injury to neural structures results from two different mechanisms. The first is the primary insult that is resulting from the mechanical event itself and the other is the secondary insult that is resulting mainly from the inflammatory response of the organism's to the primary insult. Neutrophils are blamed for orchestration of secondary injury by releasing various types of enzymes, reactive oxygen species and proinflammatory factors. These factors play important roles in glio-neuronal cell damage1,2,19,25,26,27,30).
All experiments were approved by our Institutional Review Board and performed in accordance with the local guidelines to minimize animal discomfort.
Thirty two male adult Wistar rats weighing between 250 and 300 g were used in this study. Animals were kept under constant laboratory conditions. After spinal cord trauma, manual emptying of the bladders was performed for the injured animals.
The experimental methodology used in this study was described in detail elsewhere32). Briefly, after the period of habituation, rats were randomly divided into four groups. Induction of anesthesia was performed with proper administration of ketamine hydrochloride (50 mg/kg, Ketalar, Pfizer, Istanbul, Turkey) and xylazine (10 mg/kg, Rompun, Bayer, Istanbul, Turkey). Their midbacks were prepared in a sterile fashion. Using surgical microscope, a midline incision was performed over T5 and T12 vertebrae. A total laminectomy was performed over the segments of T7 and T10 with utmost care preventing SCI. The dura was left intact. Using an aneurysm clip (70 g closing force, Yasargil FE 721, Aesculap, Istanbul, Turkey) compression was applied to the spinal cord at the midpoint of the laminectomy site. The clip was removed after 1 minute of application and the wound is irrigated with normal saline before closure of the layers. Rats in the control group did not receive clip compression procedure. No further treatment was applied to the animals in the trauma group after clip compression. Animals in treatment groups received 5 mg/kg subcutaneous adalimumab and infliximab respectively right after wound closure.
After 72 hours, rats were killed with overdose pentobarbital. Trauma site being at the epicenter, 2 cm length spinal cord segments were removed en block. Tissue samples were immediately stored in a freezer for malondialdehyde (MDA) assay.
Preoperative and postoperative neurological assessments were performed every day until the end of the study by an observer who is blinded to the groups. The Tarlov scoring system was used35). According to this system 5 tier scale was used to evaluate the neurological status : score 0, spastic paraplegia and no movement of the lower limbs; score 1, spastic paraplegia and slight movement of the lower limbs; score 2, good movement of the lower limbs, but inability to stand; score 3, able to stand, but unable to walk normally; score 4, complete recovery and normal gait/hopping.
The level of lipid peroxides in traumatized spinal cord tissue were measured as thiobarbituric acid-reactive material and determined using the method of Mihara and Uchiyama34). MDA has been identified as the product of lipid peroxidation that reacts with thiobarbituric acid to give a red species absorbing at 535 nm. The assay procedure for lipid peroxide in spinal cord tissue was set up as follows. Tissues were homogenized in 10 volumes (wt/vol) of cold 1.5% of KCl. One half a milliliter (0.5 mL) of homogenate was mixed with 3 mL of 1% of H3PO4 (Carlo Erba Reagnts, Cat No : 30406, Val de Reuil, France) and 1 mL of 0.6% of thiobarbituric acid (Merck & Co., Cat No : M.108180.0025, White House Station, NJ, USA). The mixture was then heated in boiling water for 60 minutes. After cooling, the color was extracted into 4 mL n-butanol (Sigma-Aldrich, Cat No : 24124, St. Louis, MO, USA), and the absorbance was recorded at 535 and 520 nm with a spectrophotometer (U-2900, Hitachi High Tech, Tokyo, Japan). Using tetramethoxypropane as the standard, tissue lipid peroxide levels were calculated as nanomoles per gram of wet tissue32).
Commercially available software package SPSS (SPSS Inc., Released 2007, SPSS for windows, version 16.0., Chicago, IL, USA) was used to compare MDA levels between groups. Results are expressed as mean values and probability level less than 0.05 was accepted as significant. Nonparametric tests of Kruskal-Wallis and Mann-Whitney U were used to determine differences between groups regarding mean MDA levels.
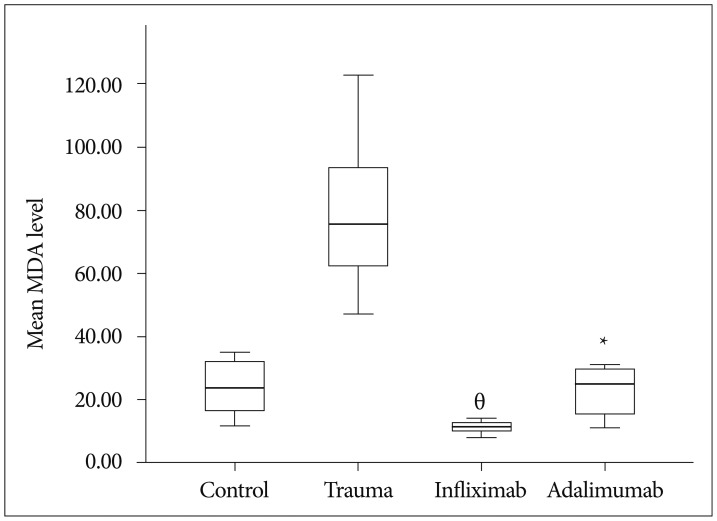
The mean MDA level of the control group was 23.87±8.96 (range : 12.00-35.00), trauma group was 79.12±24.19 (range : 47.00-123.00), infliximab group was 11.25±1.90 (range : 8.00-14.00) and adalimumab group was 22.75±8.10 (range : 11.00-31.00). MDA levels was statistically significantly different between groups, χ2(3)=23.063, p <0.01. Pairwise comparisons were performed using Dunn's procedure with a Bonferroni correction for multiple comparisons. MDA levels were significantly different between the trauma and infliximab groups (p <0.01) and trauma and adalimumab groups (p=0.022). Boxplot in Fig. 1 demonstrate mean MDA levels of groups.
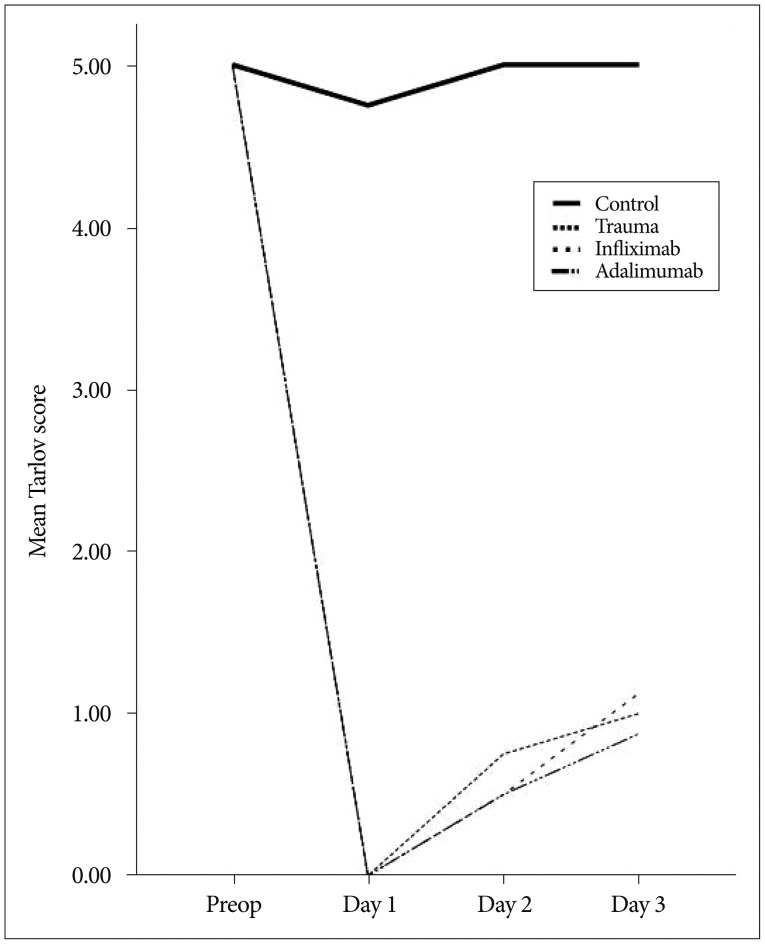
Line chart in Fig. 2 demonstrates mean Tarlov score changes throughout the experiment. There was no significant difference between trauma and treatment groups regarding Tarlov score.
Traumatic injury to the spinal cord itself creates an injury site within traumatic tissue. This is the primary injury and apart from preventive measures aimed to prevent accidents, there is virtually nothing that can be done in terms of treatment for now. On the other hand, secondary injury resulting from the changes in the trauma milieu is the main target of research33). Secondary injury results from cascades of ischemia, edema, excitatory amino acid influx and oxidative injury4,16).
Central nervous system is considered as a relatively "immune free" environment in normal circumstances. After an insult, this changes6,16) and it was demonstrated that reducing or blocking inflammatory response may have beneficial effects6,10,21,31).
Oxidative stress occurring after trauma injures nucleic acids, proteins, carbohydrates and lipids17). Since biological membranes constitute of lipids, lipid peroxidation targets them17,22). The lipid rich environment of nervous tissue makes it susceptible to such injuries. MDA is a three carbon, low molecular weight aldehyde which is considered as an important lipid peroxidation marker. Levels of MDA increases in several diseases22,31) and SCI20,24,32).
TNF-α which is a proinflammatory cytokine produced by monocytes, macrophages and T lymphocytes was proven to play important roles in various diseases such as rheumatoid arthritis, psoriatic arthritis, Crohn's disease, psoriasis and ankylosing spondylitis13,29). Blockage of TNF-α causes immune system suppression18) and this effect is used to treat aforementioned diseases13,14,18,29,36). Besides its roles in such diseases TNF-α has demonstrated roles in neural tissue damage and neuroinflammation5,23,38,39). Harrington et al.28) have demonstrated an increase in TNF-α levels in cerebrospinal fluid in the first hour of SCI. Wang et al.39) had similar findings in traumatized spinal cord tissue. Authors of these studies concluded that TNF-α has important roles in pathophysiology of traumatic SCI.
Etanercept, infliximab, adalimumab, golimumab, and certolizumab are the anti-TNF-α agents that are authorized for treatment of diseases mediated by TNF-α15). Only infliximab32) and etanercept3) has been evaluated in SCI and their favorable effects have been demonstrated. This is the first study evaluating the effects of adalimumab on SCI. The study is built on the hypothesis that TNF-α blockage by adalimumab may have beneficial effects in SCI as demonstrated in previous studies of different agents targeting TNF-α. Results here have showed that adalimumab has favorable effects on lipid peroxidation in spinal cord injured rats. The decrease in MDA levels was significant when compared to controls; however, there were no significant difference between adalimumab and infliximab. This suggests, these two agents have similar effects on lipid peroxidation levels in experimental SCI. On the other hand we could not demonstrate any favorable effect in neurological status. This may be the result of 72 hours of evaluation. This study is primarily designed to demonstrate biochemical effects of the agents on SCI and longer periods may reveal differences.
References
1. Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978; 39:236–253. PMID: 713489.
2. Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978; 39:254–266. PMID: 713490.
3. Bayrakli F, Balaban H, Ozum U, Duger C, Topaktas S, Kars HZ. Etanercept treatment enhances clinical and neuroelectrophysiological recovery in partial spinal cord injury. Eur Spine J. 2012; 21:2588–2593. PMID: 22526707.

4. Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002; 137:37–47. PMID: 12440358.
5. Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999; 16:851–863. PMID: 10547095.

6. Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma. 1992; 9(Suppl 1):S83–S91. PMID: 1588634.
7. Bracken MB. Methylprednisolone and acute spinal cord injury : an update of the randomized evidence. Spine (Phila Pa 1976). 2001; 26(24 Suppl):S47–S54. PMID: 11805609.
8. Bracken MB. Methylprednisolone in the management of acute spinal cord injuries. Med J Aust. 1990; 153:368. PMID: 2233469.

9. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012; 1:CD001046. PMID: 22258943.

10. Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984; 251:45–52. PMID: 6361287.

11. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322:1405–1411. PMID: 2278545.

12. Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985; 63:704–713. PMID: 3903070.

13. Burmester GR, Mease P, Dijkmans BA, Gordon K, Lovell D, Panaccione R, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009; 68:1863–1869. PMID: 19147611.

14. Burness CB, Keating GM. Adalimumab : a review of its use in the treatment of patients with ulcerative colitis. BioDrugs. 2013; 27:247–262. PMID: 23580096.

15. Caminero A, Comabella M, Montalban X. Tumor necrosis factor alpha (TNF-α), anti-TNF-α and demyelination revisited: an ongoing story. J Neuroimmunol. 2011; 234:1–6. PMID: 21474190.

16. Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998; 151:77–88. PMID: 9582256.

17. Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009; 157:1–11. PMID: 18977338.

18. Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children : forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010; 62:2517–2524. PMID: 20506368.

19. Ducker TB, Kindt GW, Kempf LG. Pathological findings in acute experimental spinal cord trauma. J Neurosurg. 1971; 35:700–708. PMID: 5000662.

20. Emmez H, Börcek AÖ, Kaymaz M, Kaymaz F, Durdağ E, Civi S, et al. Neuroprotective effects of gabapentin in experimental spinal cord injury. World Neurosurg. 2010; 73:729–734. PMID: 20934165.

21. Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006; 129(Pt 12):3249–3269. PMID: 17071951.

22. Grotto D, Maria LS, Valentini J, Paniz C, Schmitt G, Garcia SC, et al. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim Nova. 2009; 32:169–174.

23. Guadagno J, Xu X, Karajgikar M, Brown A, Cregan SP. Microglia-derived TNFα induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis. 2013; 4:e538. PMID: 23492769.

24. Guven C, Borcek AO, Cemil B, Kurt G, Yildirim Z, Ucankus NL, et al. Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci. 2010; 17:1563–1567. PMID: 20817464.

25. Hall ED. Inhibition of lipid peroxidation in CNS trauma. J Neurotrauma. 1991; 8(Suppl 1):S31–S40. discussion S41. PMID: 1920459.
26. Hall ED, Braughler JM. Free radicals in CNS injury. Res Publ Assoc Res Nerv Ment Dis. 1993; 71:81–105. PMID: 8380240.
27. Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M, Kawai Y, et al. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem. 1996; 66:1525–1531. PMID: 8627308.

28. Harrington JF, Messier AA, Levine A, Szmydynger-Chodobska J, Chodobski A. Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J Neurotrauma. 2005; 22:919–928. PMID: 16083358.

29. Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2009; 131:308–316. PMID: 19188093.

30. Klebanoff SJ, Vadas MA, Harlan JM, Sparks LH, Gamble JR, Agosti JM, et al. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986; 136:4220–4225. PMID: 3009619.
31. Kurt G, Cemil B, Borcek AO, Borcek P, Akyurek N, Sepici A, et al. Infliximab administration reduces neuronal apoptosis on the optic pathways in a rabbit hydrocephalus model : a preliminary report. Br J Neurosurg. 2010; 24:275–279. PMID: 20465456.
32. Kurt G, Ergün E, Cemil B, Börcek AO, Börcek P, Gülbahar O, et al. Neuroprotective effects of infliximab in experimental spinal cord injury. Surg Neurol. 2009; 71:332–336. PMID: 18440605.

33. Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 2011; 28:1545–1588. PMID: 20146558.

34. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; 86:271–278. PMID: 655387.

35. Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD, Pappa LS, Gkrepi C, et al. Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res. 2006; 133:159–166. PMID: 16337967.

36. Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis : results of a randomised controlled trial. Gut. 2011; 60:780–787. PMID: 21209123.

37. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001; (24 Suppl):S2–S12. PMID: 11805601.

38. Tang X, Wang Y, Zhou S, Qian T, Gu X. Signaling pathways regulating dose-dependent dual effects of TNF-α on primary cultured Schwann cells. Mol Cell Biochem. 2013; 378:237–246. PMID: 23479382.

39. Wang CX, Nuttin B, Heremans H, Dom R, Gybels J. Production of tumor necrosis factor in spinal cord following traumatic injury in rats. J Neuroimmunol. 1996; 69:151–156. PMID: 8823387.

40. Yu SH, Cho DC, Kim KT, Nam KH, Cho HJ, Sung JK. The neuroprotective effect of treatment of valproic Acid in acute spinal cord injury. J Korean Neurosurg Soc. 2012; 51:191–198. PMID: 22737297.

Fig. 1
Box plot demonstrating mean MDA levels. MDA levels were significantly different between the trauma and infliximab groups (theta, p<0.01) and trauma and adalimumab groups (asterisk, p=0.022) (central line for each group indicates median, with the box showing upper and lower quartiles and the whiskers showing the range). MDA : malondialdehyde




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download