Abstract
Objective
To comparatively investigate the expression of several integrins in specimens of human bone metastases and degenerative bone tissue.
Methods
Degenerative cancellous tissue was obtained from a sample of human degenerative spine. Thirteen human specimens were obtained from metastatic spine tumors, whose primary cancer was colon cancer (n=3), hepatocellular cancer (n=3), lung cancer (n=4), and breast cancer (n=3). The expression of vimentin and integrins αv, β1, and β3 was assessed in metastatic and degenerative specimens by immunohistochemistry and real-time reverse transcription-polymerase chain reaction (qRT-PCR).
Results
Immunohistochemical staining showed that vimentin and integrin αv was broadly expressed in all tissues examined. By contrast, integrin β1 was weakly expressed only in 38.4% (5/13) of tissues. Integrin β3 was consistently negative in all cases examined. qRT-PCR analysis showed that vimentin gene expression was higher in all metastatic specimens, as compared to degenerative bone. The gene expression of integrin αv in breast specimen was significantly higher than others (p=0.045). The gene expression of integrin β1 was also higher in all metastatic specimens than in degenerative bone tissue. The gene expression of integrin β3 was variable.
Cancer is the leading cause of death with most deaths related to bone metastasis28). Bone metastases cause pain, pathological fractures, compression of the spinal cord, and life-threatening imbalances in minerals including hypercalcemia12). Because of the devastating effects of metastases, attention has shifted from the pathogenesis of primary tumors to the less-understood process of metastasis2). The metastatic process of primary tumors involves several sequential steps : dissemination, survival in transit, bone migration and engagement, metastasis formation by disseminated tumor cells, and establishment of distant macrometastases21017). The emergence of primary cancer involves cancer stem cells and genetic or epigenetic alterations58). By acquiring properties of the epithelial-mesenchymal transition (EMT), cells from the malignant primary tumors disseminate to distant organs and become invasive914). Vimentin, an intermediate filament protein, is considered a marker for EMT79). Besides, the increased expression of vimentin appears to be a precondition to EMT induction7). Several factors including blood flow, growth factors, and adhesive molecules account for the predilection of some malignant tumors for bone61223).
Malignant tumor cells also produce adhesive molecules, including integrin family members, which allow tumor cells to bind bone marrow stromal cells1314). The integrin family members are involved in the signaling pathways for adhesion, migration, and cell survival13). Studies have identified several integrins, including αv, α6, β1, and β3, in skeletal metastasis of cancers originating from epithelial cells such as breast and prostate cancers42021). However, the expression of integrins in human metastatic spine cancer has not been reported. The present study was designed to investigate the upregulation of vimentin in metastatic specimens and the potential clinical implications of integrins αv, β1, and β3 expression in human metastatic spine tumors of different primary cancer origin using immunohistochemistry and real-time reverse transcription-polymerase chain reaction (qRT-PCR).
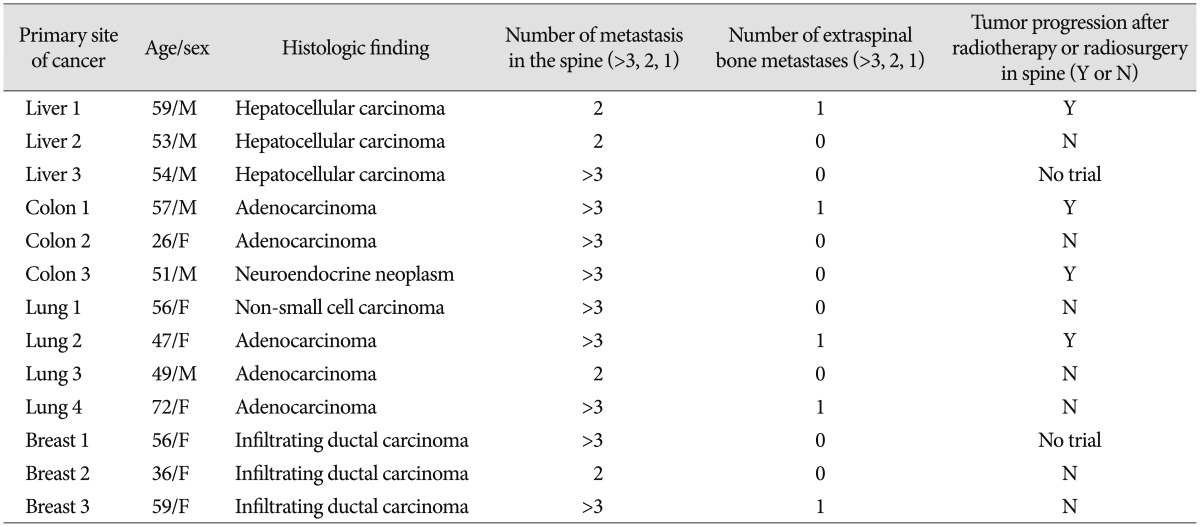
Thirteen human spinal metastatic specimens obtained from 13 consecutive patients with spine metastasis and 1 degenerative bone specimen of 1 patient with degenerative spine disease were selected from a tissue bank in our neurosurgical department. All human tissues were obtained from patients who signed a consent form allowing the collection of their tissues obtained during spine surgery in liquid nitrogen and subsequent research use. The Institutional Review Board in our hospital approved the tissue banking of specimens and their use in the present study (H1111-050-386).
The primary cancers represented by the 13 specimens were colon cancer (n=3), hepatocellular cancer (n=3), lung cancer (n=4), and breast cancer (n=3) (Table 1).
A non-metastatic bone tissue sample was obtained from the lamina of 1 patient with degenerative spine disease. Because of the limited number of trabecular tissue of degenerative bone available in the tissue bank, only 1 specimen obtained in degenerative spine was used as control. Metastatic tissue samples were obtained from the spine metastasis area during decompressive spine surgery.
Vimentin expression was assessed in the metastatic specimens in order to evaluate the malignant characteristics related to EMT. Among the adhesive molecules of the integrin family, integrin αv, β1, and β3 were selected based on the results of previous studies1321). Because the control tissue was trabecular bone and metastasis tissue was obtained from soft areas in spinal metastasis area, all specimens were not decalcified. Tissue specimens (trabecular bone and metastasis tissue) were stored in liquid nitrogen immediately after harvesting. Frozen tissue slides were prepared by cutting 10 um sections with a cryo-microtome (LEICA CM1850, Leica Biosystems, Nussloch, Germany) and mounting on slides. The slides were air dried for 30 min, fixed in cold acetone at room temperature for 5 min, removed from the acetone, and air dried again. The slides were rinsed for 5 min in tap water, incubated for 30 min in 0.3% H2O2 and 2% Triton X-100, washed in phosphate-buffered saline (PBS), incubated for 20 min in normal blocking serum, and then incubated with diluted primary antibody overnight in a cold room. The following antibodies and dilutions were used : anti-vimentin antibody (1 : 100, mouse monoclonal, Abcam, Cambrideg, UK); anti-integrin β3 antibody (1 : 100, mouse monoclonal, Novus Biologicals); anti-integrin αv antibody (1 : 500, rabbit polyclonal, Abcam); and anti-integrin β1 antibody (1 : 250, rabbit monoclonal, Abcam, Cambridge, UK). After incubation in the primary antibody, the slides were washed in PBS and incubated for 30 min with the secondary antibody (Vector, CA, USA). Vector Immpress Reagent kit anti-mouse IgG (MP7402) and anti-rabbit IgG (MP7401) were used as the secondary antibodies. The slides were washed in PBS, the tissue sections were stained for visualization with diaminobenzidine (Vector, USA), and coverslips were mounted with HistoChoice Mounting Media (Amresco, OH, USA).
The comparative expression of vimentin and integrins αv, β1, and β3 between metastatic and degenerative specimens were assessed by immunohistochemistry and qRT-PCR. The PCR primer and probe sets for vimentin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and integrins αv, β1, and β3 were obtained from Life technologies (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). Trabecular bone and metastasis tissue were stored in liquid nitrogen until RNA extraction. All specimens were treated alike. RNA was extracted from the metastatic and degenerative bone specimens with the QIAGEN RNeasy mini kit (Qiagen, LA, USA). For cDNA synthesis, 1 μg of RNA was reverse transcribed using random hexamer primers (Invitrogen, Life Technologies, CA, USA) and Impron II reverse transcriptase (Invitrogen, Life Technologies, CA, USA). The samples were analyzed using an Applied Biosystems SDS 7500 system with a master mix containing 6.25 μL water, 1.25 μL probe (2.5 M), and 12.5 μL TaqMan PCR 2× Master Mix. Fifty nanograms of reverse-transcribed total RNA in 5 μL was added as the PCR template in 96-well optical plates, and the results were analyzed on the Prism 7500 Sequence Detection System (PerkinElmer Applied Biosystems, Lincoln, CA, USA). The following PCR conditions were used. After initial activation of uracil-N-glycosylase at 50℃ for 2 min, AmpliTaq Gold was activated at 95℃ for 10 min. This was followed by 45 cycles of denaturation at 95℃ for 15 s and annealing, and extension at 60℃ for 1 min per cycle. The expression levels of the target genes were normalized to the internal control human GAPDH (FAM/MGB Probe, Primer Limited, Life Technologies, CA, USA), and fold changes were expressed relative to the data obtained at day 1.
The mean age of enrolled patients was 51.9±11.28 years. The ratio of male and female was 6 : 7. Five patients had extraspinal bone metastasis including pelvis and femur. Eleven patients underwent adjuvant radiotherapy or radiosurgery (Table 1).
Vimentin protein expression was used as a representative mesenchymal marker. Vimentin stained intensely in all samples obtained from metastatic spine tumors (Fig. 1B, C, D). Vimentin staining appeared predominantly in spindle cells and focally in epithelioid cells. Integrins are usually localized at the cell membrane of epithelial cells. Tumor cells expressed integrin αv in all cases (Fig. 1F, G, H). The integrin αv showed mostly cell membrane expression in epitheloid cells of all tumor tissue. However, integrin β1 expression was present weakly only in 38.4% (5/13) tissues (Fig. 1J, K, L); and integrin β3 was consistently negative in all cases examined (Fig. 1N, O, P).
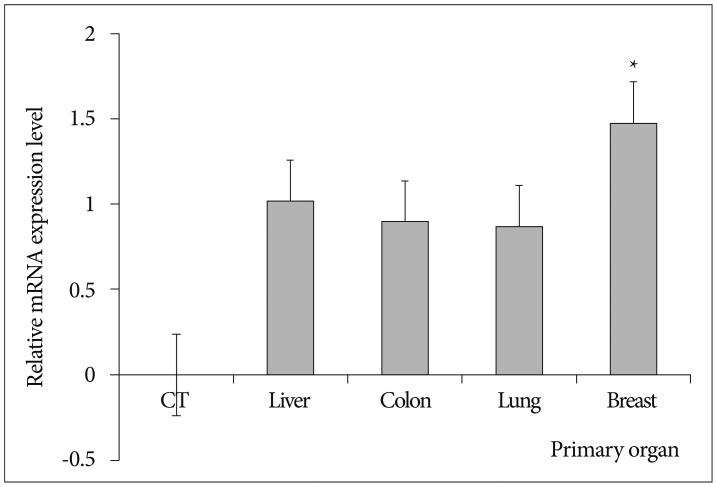
Vimentin gene expression was used as a representative EMT marker. Vimentin gene expression was higher in all metastatic specimens than in degenerative bone (Table 2, Fig. 2). The mean value of vimentin expression in metastatic specimens was 5418±8814. This result suggested that all metastatic specimens exhibited mesenchymal characteristics and would therefore be good candidates for comparative evaluation of integrin expression in degenerative bone. The mean values for integrins αv, β1, and β3 were 13±12.4, 3311±3952.4, and 5.0±6.0, respectively. All metastatic specimens had higher expression levels of integrins αv and β1, as compared with those of degenerative bone. Moreover, expression levels of integrin αv obtained from patients with breast carcinoma were significantly higher than others (p=0.045) (Fig. 3). However, the expression of integrin β3 was variable. Although the expression of integrin β3 was higher in metastatic specimens obtained from breast and colon carcinomas than those from degenerative bone, the differences between metastatic specimens and degenerative bone were less prominent, as compared with integrin αv and β1. The values were lower in other specimens from hepatocellular and lung carcinoma than degenerative bone (Table 2). However, because the comparison of integrin expression between degenerative bone and tumor tissues was relative to 1 sample of degenerative bone tissue, statistical analysis was not significant. The qRT-PCR results of vimentin, integrin αv, β1, and β3 expression in control and tumor specimens were representative of 1 of 3 independent experiments.
Thirteen human specimens were obtained from metastatic spine tumors, whose primary cancer was colon cancer, hepatocellular cancer, lung cancer, and breast cancer. The enrolled patients underwent adjuvant radiotherapy or chemotherapy. The present immunohistochemistry and qRT-PCR data showed that the expression of vimentin and integrin αv and β1 was higher in metastatic spine specimens, as compared with degenerative bone tissue.
Most malignant tumors are epithelial in origin, and malignant tumors are highly osteotropic22). Acquisition of an invasive mesenchymal phenotype is necessary for metastasis of a malignant tumor through the destruction of the epithelial side1822). Acquisition of EMT properties causes the polarized epithelial cells to become invasive migratory mesenchymal cells1922). Many EMT biomarkers, such as vimentin, N-cadherin, Snail1, FOXC2, and Twist, have been reported2224). Among these biomarkers, the intermediate filament protein vimentin is regarded as a requisite regulator of EMT induction and migration of malignant tumor cells724). Elevated expression of vimentin is regarded as a poor prognostic factor in breast cancer25). Therefore, an increase in the expression of vimentin may reflect the capacity for bone metastasis of malignant tumors. In the present study, vimentin expression level was higher in all metastatic specimens, as compared to degenerative bone suggestive of EMT in the tumor samples. The results of the present study indicated that the vimentin is an important biomarker of malignant tumor bone metastasis.
Integrins are a family of transmembrane glycoproteins that facilitate cell-to-cell and cell-to-extracelluar matrix adhesion and cell migration1113). Integrins mediate inside-out and outside-in signaling, and may trigger the integrin-mediated cell-signaling cascade in metastasis11). Malignant transformation, invasion, and metastasis to distant organs including the skeletal system, require the activity of many adhesive proteins including integrin family members. Metastatic tumor cells show differences in integrin activation, as compared with non metastatic tumor cells131626). Among the 24 integrins identified, integrins such as αvβ3, the β1 family, and β3 have been implicated in the processes involved in tumor metastasis, such as angiogenesis, adhesion, and progression13). The qRT-PCR results showed that the expression of integrins β1 and αv was higher in metastatic specimens than non metastatic tissue such as the degenerative bone specimen. Integrin αvβ3 is expressed in osteoclast cells and the selective inhibitor of integrin αvβ3 was shown to decrease bone resorption in an animal model27). The αvβ3 integrin is an adhesion receptor expressed by breast cancer cells and osteoclasts1627). The present study showed that the expression of the integrin αv in specimens of breast carcinoma increased significantly, as compared to others. Integrin β3 may be associated with angiogenesis during tumor progression and cancer invasion1315). In the present study, the expression of integrin β3 was elevated only slightly in metastatic specimens, such as those of hepatocellular carcinoma and breast cancer, as compared with degenerative bone. This suggested that in breast carcinoma, integrin αv and β3 expression but not integrin β1, may be related with tumor induced-osteolysis and angiogenesis. Therefore, integrin αv and β3 are potential therapeutic targets for prevention of spine metastasis of malignant tumor including breast carcinoma.
In the present study, the immunochemical expression of integrins β1 and β3 was weak and absent, respectively. The discrepant results between qRT-PCR and immunohistochemistry might be due to freezing of the tumor samples. Immunohistochemical results obtained from frozen tissue possibly do not reflect integrin expression accurately.
There were several limitations of the present study. First, although the bone specimens were obtained from humans, the number of specimens was small and variable types of malignant tumors were included. Therefore, the evidence may be weak in terms of providing insights into the relationships between integrins αv, β1, and β3 and the spine metastasis of malignant tumors, and any therapeutic potential of monitoring the expression of integrins. The present study measured only the expression of integrins αv, β1, and β3 using immunohistochemistry and qRT-PCR, which may not reflect the entire intracellular pathway related to integrins. Future studies of malignant tumors from more patients are needed to identify the pathological mechanisms and the relationships between integrins and malignancy. The present study also compared integrin expression between metastasized bone tissue and nonmetastatic bone tissue but not that of the primary tumor specimen. Future studies are required to compare integrin expression between malignant tumor and nonmalignant specimens, and between metastasized bone specimens and primary tumor tissues. However, because no studies have evaluated integrin expression in metastatic spine specimens, despite the limitations, the results of the present study provide meaningful information about the potential clinical implications of integrin β1 and αv for spine metastasis.
Vimentin expression a biomarker of EMT was highest in specimens obtained from spinal metastatic tumors. The higher expression of the integrins αν and β1 in metastatic specimens suggested that the integrins may be a potential target for the treatment of spine metastases from malignant tumors.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2010-0028631).
References
1. Clëzardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr Cancer Drug Targets. 2009; 9:801–806. PMID: 20025568.

2. Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol. 2010; 222:1–15. PMID: 20681009.

3. Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, et al. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001; 12:99–107. PMID: 11243469.
4. Engebraaten O, Trikha M, Juell S, Garman-Vik S, Fodstad Ø. Inhibition of in vivo tumour growth by the blocking of host alpha(v)beta3 and alphaII(b)beta3 integrins. Anticancer Res. 2009; 29:131–137. PMID: 19331142.
5. Gudadze M, Kankava K, Mariamidze A, Burkadze G. Distribution of cancer stem cells in ductal invasive carcinoma of breast (review). Georgian Med News. 2013; (222):44–50. PMID: 24099814.
6. Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986; 261:12665–12674. PMID: 3745206.

7. Ivaska J. Vimentin : central hub in EMT induction? Small GTPases. 2011; 2:51–53. PMID: 21686283.
8. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea : incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14. PMID: 23613665.

9. Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition : what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 2014; 40:341–348. PMID: 24090504.

10. Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone : a multistep process. Future Oncol. 2011; 7:1285–1297. PMID: 22044203.
11. Qin J, Vinogradova O, Plow EF. Integrin bidirectional signaling : a molecular view. PLoS Biol. 2004; 2:e169. PMID: 15208721.
13. Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis : integrating tumor cell and stromal cell interactions. Bone. 2011; 48:54–65. PMID: 20850578.

14. Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010; 3:90–99. PMID: 21139809.
15. Shen Z, Ye Y, Kauttu T, Seppänen H, Vainionpää S, Wang S, et al. Novel focal adhesion protein kindlin-2 promotes the invasion of gastric cancer cells through phosphorylation of integrin β1 and β3. J Surg Oncol. 2013; 108:106–112. PMID: 23857544.

16. Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, et al. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006; 8:R20. PMID: 16608535.
17. Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis : how the skeleton affects tumor behavior. Bone. 2011; 48:6–15. PMID: 20643235.

18. Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013; 27:2192–2206. PMID: 24142872.

19. Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009; 69:7135–7139. PMID: 19738043.

20. van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Pelger RC, van der Pluijm G. Integrin αv expression is required for the acquisition of a metastatic stem/progenitor cell phenotype in human prostate cancer. Am J Pathol. 2011; 179:2559–2568. PMID: 21907176.

21. van der Horst G, van den Hoogen C, Buijs JT, Cheung H, Bloys H, Pelger RC, et al. Targeting of αv-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011; 13:516–525. PMID: 21677875.

22. van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011; 48:37–43. PMID: 20670698.

23. van der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos S, Löwik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction : elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J Bone Miner Res. 2001; 16:1077–1091. PMID: 11393785.

24. Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011; 30:1436–1448. PMID: 21057535.

25. Yamashita N, Tokunaga E, Kitao H, Hisamatsu Y, Taketani K, Akiyoshi S, et al. Vimentin as a poor prognostic factor for triple-negative breast cancer. J Cancer Res Clin Oncol. 2013; 139:739–746. PMID: 23354842.

26. Yoneda T. Cellular and molecular basis of preferential metastasis of breast cancer to bone. J Orthop Sci. 2000; 5:75–81. PMID: 10664443.

27. Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007; 67:5821–5830. PMID: 17575150.

Fig. 1
Immunohistochemstry of tumor samples. The result of immunohistochemical staining. A : Control bone stroma, vimentin. B : Metastatic breast cancer, vimentin. C : Metastatic colon cancer, vimentin. D : Metastatic hepatocellular carcinoma, vimentin. E : Normal bone stroma, αv. F : Metastatic breast cancer, αv. G : Metastatic colon cancer, αv. H : Metastatic hepatocellular carcinoma, αv. I : Normal bone stroma, β-1. J : Metastatic breast cancer, β-1. K : Metastatic colon cancer, β-1. L : Metastatic hepatocellular carcinoma, β-1. M : Normal bone stroma, β-3. N : Metastatic breast cancer, β-3. O : Metastatic colon cancer, β-3. P : Metastatic hepatocellular carcinoma, β-3. Magnification of all slides (×400).
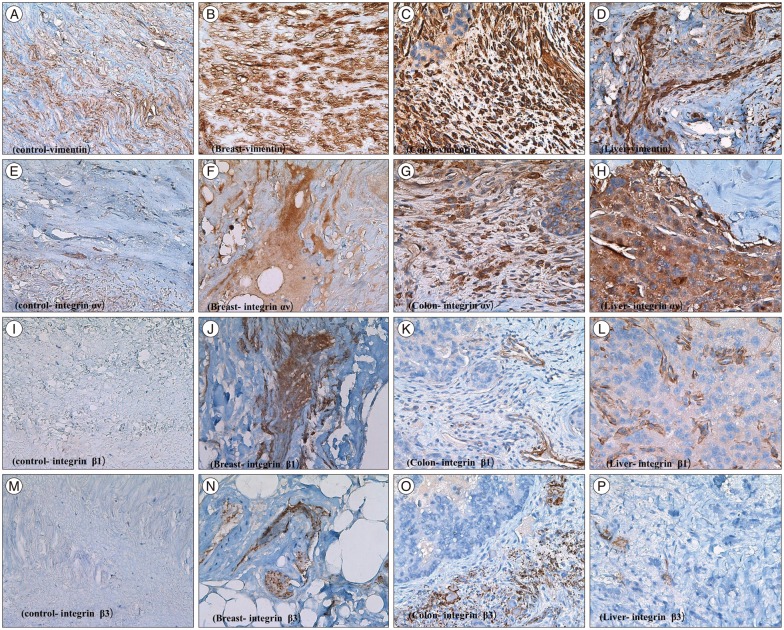
Fig. 2
Vimentin mRNA expression by qRT-PCR in degenerative and metastatic specimens. The values of mRNA in all metastatic specimens were expressed relative value 1 of the degenerative bone. However, this figure shows the logarithmic scale of vimentin mRNA expression in degenerative bone and all metastatic spinal specimens. Therefore, the value of mRNA in all metastatic specimens expressed relative value 0 of the degenerative bone in this figure. The mRNA value of was increased in all metastatic specimens, as compared to degenerative bone tissue. CT : control, degenerative bone, H : hepatocelluar carcinoma, Co : colon cancer, L : lung cancer, B : breast cancer, qRT-PCR : real-time reverse transcription-polymerase chain reaction.
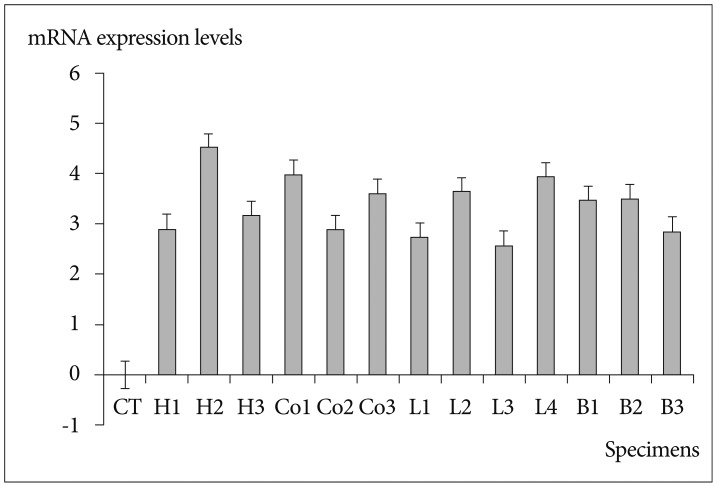
Fig. 3
Integrin αv mRNA expression by qRT-PCR in degenerative and metastatic specimens. Integrin αv mRNA in all metastatic specimens were expressed relative to value 1 of the degenerative bone. However, this figure shows the logarithmic scale of the mRNA expression levels of integrin αv in degenerative bone and all metastatic specimens. Therefore, the value of mRNA in all metastatic specimens expressed relative value 0 of the degenerative bone in this figure. The mean values integrin αv in breast specimens were significantly higher than those of others (p=0.045). *p<0.05. CT : control and degenerative bone, qRT-PCR : real-time reverse transcription-polymerase chain reaction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download