Abstract
Objective
Covered stent has been recently reported as an effective alternative treatment for direct carotid cavernous fistulas (DCCFs). The purpose of this study is to describe our experiences with the treatment of DCCF with covered stents and to evaluate whether a covered stent has a potential to be used as the first choice in selected cases.
Methods
From February 2009 through July 2013, 10 patients underwent covered stent placement for a DCCF occlusion. Clinical and angiographic data were retrospectively reviewed.
Results
Covered stent placement was performed for five patients primarily as the first choice and in the other five as an alternative option. Access and deployment of a covered stent was successful in all patients (100%) and total occlusion of the fistula was achieved in nine (90%). Complete occlusion immediately after the procedure was obtained in five patients (50%). Endoleak persisted in five patients and the fistulae were found to be completely occluded by one month control angiography in four. The other patient underwent additional coil embolization by a transvenous approach. Balloon inflation-related arterial dissection during the procedure was noted in two cases; healing was noted at follow-up angiography. One patient suffered an asymptomatic internal carotid artery occlusion noted seven months post-treatment.
Direct carotid cavernous fistula (DCCF) results from head and facial injuries or cavernous internal carotid artery (ICA) aneurysm rupture. The direct communication between the ICA and the cavernous sinus produces a series of symptoms including exophthalmos, conjunctival injection, bruit, and cranial nerve impairment.
At present, the endovascular approach is the mainstay for treatment of a DCCF. The treatment options include : detachable balloons, coils, other embolic material, and stents through a transarterial or transvenous approach7,8,13,14). In recent years, covered stents have been applied for the treatment of DCCF with encouraging short-to-midterm clinical results, thus, showing that a covered stent is an effective alternative after failure of conventional endovascular therapy5,6). However, there are few reports regarding the application of covered stents in DCCF treatment as the first choice. The purpose of this study was to evaluate whether covered stent placement could be a preferred first-line method for DCCF patients. We present 10 cases of DCCF treated with covered stents; in five, a covered stent was the first choice.
From January 2009 through July 2013, a total of 13 cases of DCCF were treated by the endovascular approach at our institution. Therapeutic alternatives were discussed with both neurosurgical and neurointerventional teams in a multidisciplinary decision-making process. A Jostent coronary stent graft (Abbott Vascular, Rangendingen, Germany) was applied in 10 patients with DCCF (six females and four males; mean age, 45.8±16.1 years). Head trauma was the cause of DCCF in seven patients; however, two patients did not have a history of a head injury before the onset of the fistula. The fistula developed after a Le Fort II procedure for open-bite malocclusion in the remaining patient. Nine patients presented with symptoms, such as tinnitus, diplopia, conjunctival injection, and proptosis with alert mentality. The other patient was in a vegetative state due to severe brain injury, and the CCF was found in a follow-up brain CT scan. A covered stent placement was performed in five patients primarily as the first choice and in the other five as an alternative option after incomplete occlusion with coils. Three of the latter patients were transferred from another facility after treatment failure; they underwent covered stent placement as the first treatment option at our institution. Clinical and angiographic features are summarized in Table 1. This study was conducted with the approval of the Institutional Review Board of our hospital.
All procedures were performed under local anesthesia. The angio-architectures were evaluated by using the Integris V (Philips Medical System, Best, the Netherlands) biplane system, including three-dimensional (3D) rotational angiography. Angiographic evaluation included ipsilateral and contralateral carotid and vertebral angiographies with neck (carotid) compression. The coronal source images obtained from 3D rotational angiography were also reviewed. Angiographic segments of the cavernous ICA to demonstrate the fistula point were classified as follows : vertical, genu, and horizontal portion.
Prior to the interventional procedures, most of the patients were given dual antiplatelet medication. Heparin (3000 IU) was administered as a bolus after femoral arterial sheath placement and intermittently thereafter 1000 IU bolus per hour with monitoring of activated clotting time to maintain 250-300 seconds.
After arterial access, a double coaxial guide system was used. A 7-F Shuttle (Cook, Bloomington, IN, USA) was advanced to the level of the common carotid artery, and then a 7-F Softip guide catheter (Boston Scientific, Natick, MA, USA) was advanced coaxially into the cervical ICA near the carotid canal. After selection of the middle cerebral artery with the aid of a microcatheter through the true lumen of the cavernous ICA, the covered stent was placed at the fistula point of the cavernous ICA along an exchange microguidewire. Then, the stent was released with staged-ballooning. At first, the stent was deployed by low-pressure balloon inflation until the balloon-waist disappeared. Then, the balloon inflation was repeated with gradually-increasing pressure to ensure good apposition of the stent to the vessel wall; the balloon was gently moved into the proximal position to avoid arterial dissection. If endoleak with large amount persisted despite balloon inflation up to the bursting pressure, a larger balloon was found to be helpful for reduction of the endoleak.
Dual antiplatelet medication was maintained for at least three month after the procedure, and a single agent was maintained for at least one year.
The degree of DCCF occlusion was assessed by completion angiography with a 3-point scale : total occlusion (no residual shunt), near-total occlusion (minimal residual shunt), and subtotal occlusion (high flow residual shunt).
In patients with total occlusion of the fistula, clinical follow-up was recommended at one and three months after the procedure. Only if the patient showed aggravation of the clinical symptoms, imaging follow-up, such as DSA or MRA, was recommended. In patients with near-total or subtotal occlusion after the procedure, a follow-up DSA was recommended one month post procedure to confirm the progressive occlusion, or to decide if further treatment was necessary.
Covered stents were implanted in five patients (50%) as the first choice and in the other five patients (50%) after failure of transarterial embolization (n=4) or transvenous coil embolization (n=1). Stent delivery and deployment was technically successful in all cases. Total occlusion at the conclusion of the procedure was obtained for five patients (50%), near-total occlusion was for three, and subtotal occlusion was for the other two. Most patients receiving covered stents as the alternative option had total occlusion immediately after procedure, whereas four patients treated with covered stents as the first choice had a remnant fistula due to endoleak. All four patients with endoleak underwent one month control angiography, and complete occlusion was demonstrated in three. The remaining patient experienced increased shunt flow; additional coil embolization via transvenous approach was performed. The other patients were improved without evidence of fistula recurrence for clinical and radiologic follow-up period (range, 1-21 months; mean 5.3 months). Minimal stenosis at the proximal end of stent was detected in three cases (Fig. 1). In regard to procedure-related complications, balloon inflation-related arterial dissection occurred in two patients; it was noted to have healed at the follow-up angiography. One case of asymptomatic internal carotid artery occlusion was identified at seven month follow-up CT angiography.
A 30-year-old man with abducens nerve palsy and pulsatile tinnitus was admitted for treatment of DCCF, which developed after open reduction of a medial orbital wall fracture caused by blunt trauma. The fistula was located at the genu portion of the cavernous ICA. With a 7-F Shuttle flexor and 7-F guiding catheter co-axial system, a 2.3-F microcatheter was introduced into the left middle cerebral artery. Then, a covered stent (5.0×19 mm) was advanced to the fistula location and repeated balloon-dilatation was performed with gradually increasing pressure. Endoleak persisted; however, shunt flow markedly decreased. Balloon inflation-induced arterial dissection developed at the distal portion of the stented segment. After confirmation of ICA patency and absence of thrombus, the procedure was completed. At one-month control angiography, the fistula was completely occluded and the dissection was stable. Minimal stenosis at the proximal end of stent was detected; however, patency of the stented ICA was maintained. The patient fully recovered (Fig. 1).
A 61-year-old woman was admitted for endovascular treatment of a DCCF. She had diplopia due to oculomotor and abducens nerve palsies. On conventional angiography, the fistula was located at the vertical segment of cavernous ICA. After placement of a covered stent (4.5×19 mm) at the fistula location, the balloon was gradually inflated until its waist disappeared. Then, repeated inflation of the balloon with higher pressure was carried out. Although the flow of the shunt was notably decreased, endoleak remained. Additional balloon inflation was performed with a larger caliber balloon (5.0×12 mm), and the fistula was completely occluded. The patient was discharged the day after the procedure without complications (Fig. 2).
DCCF is frequently associated with poor neurological outcomes, necessitating surgical or endovascular repair. Due to its technical difficulty and associated morbidity, direct surgical closure has now been supplanted by endovascular methods.
Following its introduction in the 1970s, occlusion with a detachable balloon quickly became the method of choice to treat DCCF. Currently, the detachable balloon is still considered as the optimal therapeutic tool for DCCF4,13). However, balloons do have some limitations : the fistula and cavernous sinus must be of appropriate size, premature deflation can result in fistula recurrence, and mass effect might occur7). An additional shortcoming is their limited availability. Detachable or pushable coils have disadvantages similar to those of balloons : mass effect, fistula recurrence due to coil compaction, and higher cost10).
During the past decade, the endovascular stent graft has been widely used for placement in the aorta, peripheral arteries and coronary arteries2,3). Recently, covered stents have been utilized in the treatment of intracranial aneurysms and DCCF as well6,15). A series of reports have been published regarding the feasibility, safety, and outcome of covered stents in the treatment of DCCF1,11,16,17); thus, showing that a covered stent can be an effective and reliable alternative method. In our series of 10 cases treated with covered stents, the technical success rate was 100%. Complete fistula occlusion was obtained in nine cases (90%). According to the literatures, the technical success rate and complete occlusion rate at the conclusion of the procedure with a covered stent for DCCF ranges 90-100% and 75-100%, respectively1,11,16,17). Our results are similar to those reports. One of the main advantages of graft stents may be that they do not exert any mass effect on the cranial nerves thus may enhance cranial nerve symptom recovery.
Given the limited availability of detachable balloons, we believe that covered stent placement could be a first-line method, as shown in five cases in our series. A covered stent immediately obliterates DCCF in a single step by placing an impermeable barrier at the site of abnormal communication. In addition, it decreases the risk of ischemic stroke by preserving the ICA patency, as compared to carotid trapping, which is the last resort in the treatment of DCCF. Treating DCCF with covered stents appears to be more reasonable because it does not require deployment of any embolization materials in the cavernous sinus that may complicate the procedure and enhance its associated risk. To the best of our knowledge, only Gomez et al.6) has reported two cases of a covered stent as the first choice, with complete fistula occlusion and good ICA patency at 7-month follow-up. In our stent-as-the-first-choice group, the total occlusion rate was 80% (4/5), including a patient with total occlusion immediately following the procedure and three patients with progressive occlusion noted at one month follow-up. Results of Gomez et al.6) and our experience show that a covered stent has the potential to be used as the first choice in DCCF treatment.
Endoleak, defined as persistent perfusion of the space between the stent graft and the parent vessel wall, is the most common procedural failure of endovascular repair. Hoit et al.9) reported their experience with a covered stent for DCCF and a traumatic pseudoaneurysm. According to the report, transient endoleak occurred in 83% (5/6); the endoleaks were related to poor stent vessel apposition or size mismatch. In two patients, they were able to completely appose the stent to the vessel wall with an additional angioplasty; however, three patients required deployment of an additional graft stent. Saatci et al.15) found that 32% (8/25) had an endoleak immediately after intracranial covered stent deployment for aneurysm treatment. However, 75% (6/8) of the endoleaks disappeared by balloon re-inflation at the proximal and distal ends of the stent grafts. In our series, transient endoleaks were observed in 67% (6/9) immediately after stent deployment. To decrease the flow of the endoleak, we applied two methods in consecutive order : 1) repeated-dilation with gradually-increasing pressure (initially), and 2) use of a balloon with a larger caliber (if endoleak persisted). The former method induced successful occlusion in three cases, and subtotal occlusion with remarkably decreased shunt flow with remnant in one case. The latter method also induced the similar results. Both methods were effective to decrease or halt shunt flow, improving the approximation of the stent to the fistula point.
Although four out of five cases with endoleak immediately after the procedure were found to have progressive occlusion at follow-up, the endoleak acted as a structural limitation to the covered stent. All of the endoleak, which persisted after completion of the procedure, were located at the posterior genu portion of cavernous ICA. The curvature of the posterior genu, combined with the limited flexibility of covered stent, may lead to poor apposition of the stent to the vessel wall, especially at the lesser curve of covered stent. In addition, this sectional shape mismatch might cause stent migration and incomplete coverage of the fistula during inflation; thus, enhancing the risk of endoleak, for which the staged ballooning technique mentioned above, offers a feasible solution. Of course, the endoleak may also be caused by a size mismatch between the covered stent and the parent vessel due to situation such as high flow-induced parent artery remodeling, transient vasospasm, and rupture of the covered membrane1,9). In a large series of intracranial stent grafting, Zhu et al.19) noted that the number, diameter and angulation of stents are possible predictors of an endoleak. Recovery methods for endoleak elimination consist of re-dilation, a second covered or bare stent9,15), trans-venous coil embolization, and observation, which offer an opportunity for spontaneous occlusion of endoleaks with minimal slow residual filling16).
Other important points regarding Jostent stent graft are long-term patency of the stented ICA and difficulty of stent delivery in a tortuous intracranial artery. The covered stent is composed of a tubular expanded polytetrafluoro-ethylene (ePTFE) layer sandwiched between two stainless steel stents. Due to this composition and construction, rigidity is the main shortcoming of this stent. It is difficult to adapt a rigid stent designed for coronary use to the curves of the neurovascular anatomy; this situation is associated with the poor navigation of the stent graft in the cerebral arteries. Possible problems that may result from this rigidity are dissection and vasospasm of the cerebral arteries, or even endoleaks due to sectional shape mismatch between the stent and parent artery. However, technical success can be achieved with no harm to the patient, if necessary precautions are taken both in case selection and during the procedure by using necessary interventional adjunctive tools and techniques such as a coaxial guiding system and use of the buddy wire to enhance the guidance support. In regards to the patency of the stented ICA, encouraging results for intracranial covered stent have been reported in the literature, ranging 83.3% to 100%1,6,15,16,17). Minimal stenosis at proximal end of stent was noted in three cases in our series. A similar finding was reported by Lukito et al.12); they attributed it to the incomplete coverage of the proximal and distal parts of the stent by the PTFE. In addition, the rigidity of the covered stent may cause vessel trauma at the edge during implantation and intimal hyperplasia as a response. Therefore, long-term antiplatelet maintenance is mandatory after procedure. In our series, the stented ICA was found to be occluded in one patient on seven month follow-up, despite the fact that it was a subclinical event. The patient maintained antiplatelet medication with good compliance and responded well to clopidogrel.
The group using the covered stent as an alternative option were found to have better results than the 'the first choice group' in terms of immediate morphological outcome. The better outcome in the former group may be related to a decrease in shunt flow following initial treatment. However, we believe that the covered stent can be used as the first-line therapeutic tool in selected DCCF patients because the residual endoleak with markedly decreased shunt flow will heal spontaneously or become manageable if it persists. The decision to treat DCCF with covered stents as the first line therapy is based on several factors : the non-availability of detachable balloon; the high cost and risk associated with coil embolization; and the presence of a favorable carotid siphon anatomy with a less tortuous course. Recently, a new kind of covered stent specifically designed for intracranial use has reduced on the problem. The stent is reported to be more flexible and to achieve good preliminary results in DCCF treatment18). Further progress can be anticipated as technology of the covered stents and their delivery systems continue to evolve. Stent-based artery reconstruction could soon become the first-line therapy for DCCF as experience with this device increases, materials continue to improve, and more data are accumulated.
Our experience shows that a covered stent graft can serve as an effective and safe micro-invasive technique in DCCF treatment. It has the potential to be used as first-line therapy in selected cases because it may offer a more definitive reconstructive treatment than the conventional endovascular approach. However, in regard to for the limited number of case series, large-scale randomized studies are warranted to further develop the specifications and indications of this device.
References
1. Archondakis E, Pero G, Valvassori L, Boccardi E, Scialfa G. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007; 28:342–347. PMID: 17297009.
2. Briguori C, Sarais C, Sivieri G, Takagi T, Di Mario C, Colombo A. Polytetrafluoroethylene-covered stent and coronary artery aneurysms. Catheter Cardiovasc Interv. 2002; 55:326–330. PMID: 11870936.

3. Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994; 331:1729–1734. PMID: 7984192.

4. Debrun G, Lacour P, Vinuela F, Fox A, Drake CG, Caron JP. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981; 55:678–692. PMID: 6458669.

5. Gemmete JJ, Ansari SA, Gandhi DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol. 2009; 29:62–71. PMID: 19458580.

6. Gomez F, Escobar W, Gomez AM, Gomez JF, Anaya CA. Treatment of carotid cavernous fistulas using covered stents : midterm results in seven patients. AJNR Am J Neuroradiol. 2007; 28:1762–1768. PMID: 17885249.

7. Gupta AK, Purkayastha S, Krishnamoorthy T, Bodhey NK, Kapilamoorthy TR, Kesavadas C, et al. Endovascular treatment of direct carotid cavernous fistulae : a pictorial review. Neuroradiology. 2006; 48:831–839. PMID: 16969673.

8. Halbach VV, Higashida RT, Barnwell SL, Dowd CF, Hieshima GB. Transarterial platinum coil embolization of carotid-cavernous fistulas. AJNR Am J Neuroradiol. 1991; 12:429–433. PMID: 2058488.
9. Hoit DA, Schirmer CM, Malek AM. Stent graft treatment of cerebrovascular wall defects : intermediate-term clinical and angiographic results. Neurosurgery. 2008; 62(5 Suppl 2):ONS380–ONS388. discussion ONS388-ONS389. PMID: 18596518.
10. Klisch J, Huppertz HJ, Spetzger U, Hetzel A, Seeger W, Schumacher M. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae : results for 31 patients and review of the literature. Neurosurgery. 2003; 53:836–856. discussion 856-857. PMID: 14519216.
11. Li J, Lan ZG, Xie XD, You C, He M. Traumatic carotid-cavernous fistulas treated with covered stents : experience of 12 cases. World Neurosurg. 2010; 73:514–519. PMID: 20920935.

12. Lukito G, Vandergoten P, Jaspers L, Dendale P, Benit E. Six months clinical, angiographic, and IVUS follow-up after PTFE graft stent implantation in native coronary arteries. Acta Cardiol. 2000; 55:255–260. PMID: 11041124.

13. Luo CB, Teng MM, Yen DH, Chang FC, Lirng JF, Chang CY. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma. 2004; 56:1214–1220. PMID: 15211128.

14. Morón FE, Klucznik RP, Mawad ME, Strother CM. Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol. 2005; 26:1399–1404. PMID: 15956506.
15. Saatci I, Cekirge HS, Ozturk MH, Arat A, Ergungor F, Sekerci Z, et al. Treatment of internal carotid artery aneurysms with a covered stent : experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol. 2004; 25:1742–1749. PMID: 15569740.
16. Tiewei Q, Ali A, Shaolei G, Feng L, Zhongsong S, Xuesong L, et al. Carotid cavernous fistulas treated by endovascular covered stent grafts with follow-up results. Br J Neurosurg. 2010; 24:435–440. PMID: 20515263.

17. Wang C, Xie X, You C, Zhang C, Cheng M, He M, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2009; 30:1342–1346. PMID: 19342540.

18. Wang YL, Ma J, Ding PX, Li YD, Han XW, Wu G. Treatment of post-traumatic carotid-cavernous fistulas with the Willis covered stent. A preliminary prospective study. Interv Neuroradiol. 2012; 18:172–177. PMID: 22681732.

19. Zhu YQ, Li MH, Lin F, Song DL, Tan HQ, Gu BX, et al. Frequency and predictors of endoleaks and long-term patency after covered stent placement for the treatment of intracranial aneurysms : a prospective, non-randomised multicentre experience. Eur Radiol. 2013; 23:287–297. PMID: 22782569.

Fig. 1
A : Left ICA angiography shows that steal of the flow toward the cavernous sinus and primary flow drained into ophthalmic vein, superior petrosal sinus, and inferior petrosal sinus. B : Left ICA angiography performed under ipsilateral carotid compression shows that the fistula was located at the genu portion of the cavernous ICA. C : Coronal source image of 3D rotational angiography shows the connection between the ICA and the cavernous sinus. D : Repeated balloon inflation with higher pressure to augment apposition of the stent to the vessel wall was performed. E : Final angiography shows that the fistula with decreased shunt flow persisted and balloon-induced arterial dissection was observed. F : The fistula was completely occluded and arterial dissection was stable at one month follow-up angiography. Minimal stenosis at the proximal end of stent was detected. ICA : internal carotid artery, 3D : three-dimensional.
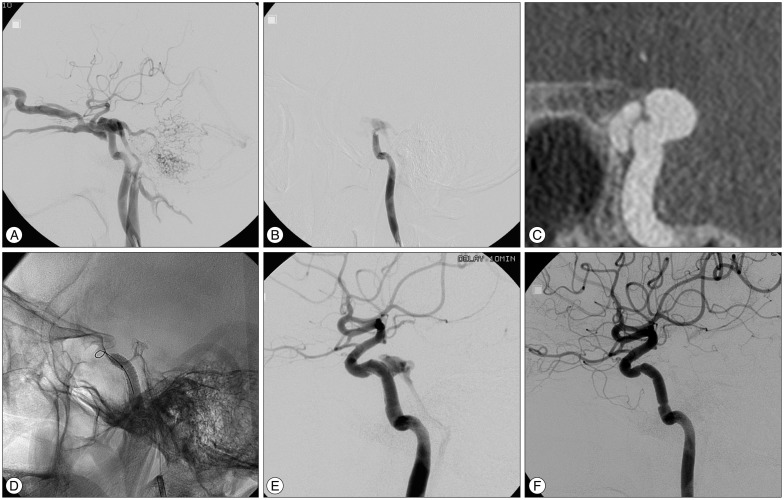
Fig. 2
A : Right ICA angiography shows that steal of the flow toward the cavernous sinus and intracranial flow was diminished. B : Left ICA angiography performed under ipsilateral carotid compression shows that the fistula was located at the vertical portion of the cavernous ICA. C : After placement of the covered stent at the fistula location, initial balloon inflation was performed to detach the covered stent from the balloon. D : High shunt flow remained; however, velocity was lessened. E : Repeated balloon inflation with higher pressure was carried out. F : The endoleak with more decreased shunt flow remained. G : Additional balloon inflation with a larger sized-balloon was performed. H : The fistula was totally occluded. ICA : internal carotid artery.
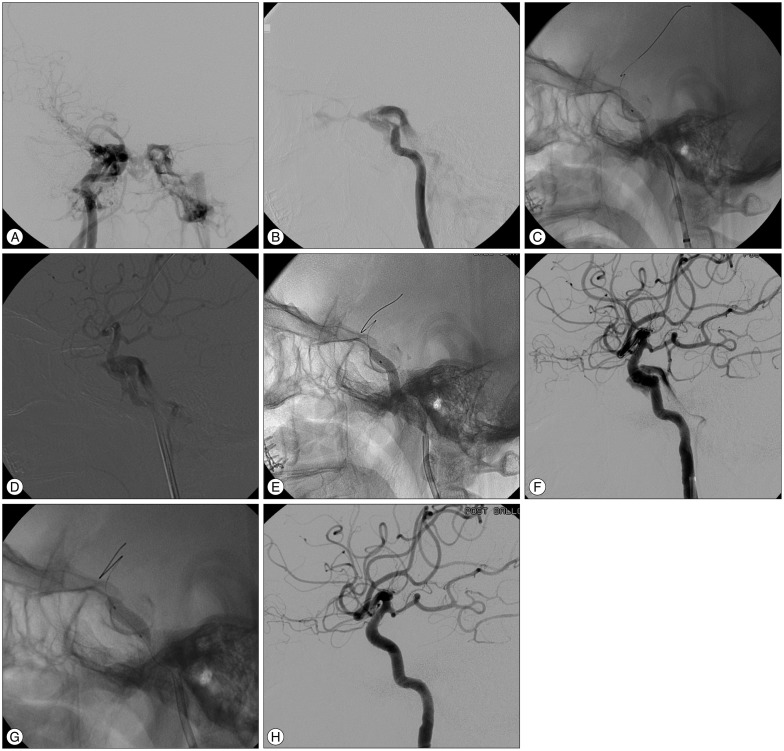
Table 1
Summary of the patients' data
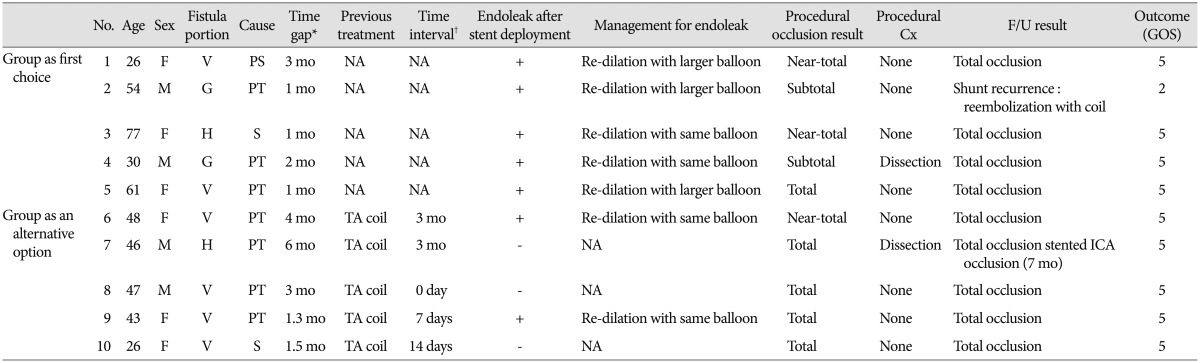
*Time gap means the interval between initial trauma (surgery) and treatment with covered stent, †Time interval means the interval between previous treatment and treatment with covered stent. F : female, M : male, H : horizontal, G : genu, V : vertical, PS : post-surgery, PT : post-trauma, S : spontaneous, mo : month, NA : non-applicable, TA : trans-arterial, TV : trans-venous, Cx : complication, F/U : follow-up, GOS : Glasgow outcome scale, ICA : internal carotid artery




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download