Abstract
A 59-year-old female presented with progressive right proptosis, chemosis and ocular pain. An imaging work-up including conventional catheter angiography showed a right-sided dural arteriovenous fistula of the cavernous sinus, which drained into the right superior petrosal sinus, right superior ophthalmic vein, and right inferior ophthalmic vein, and cortical venous reflux was seen via the right petrosal vein in the right posterior fossa. After failure of transvenous embolization, the patient underwent Gamma Knife radiosurgery (GKRS). At one month after GKRS, she developed increasing ocular pain and occipital headache. Repeat angiography showed partial obliteration of the fistula and loss of drainage via the superior and inferior ophthalmic veins with severe congestion, resulting in slow flow around the right cerebellar hemisphere. Prompt transarterial embolization relieved the patient's ocular symptoms and headache. We report on a case of paradoxical exacerbation of symptoms resulting from obstruction of the venous outflow after GKRS for treatment of a dural arteriovenous fistula of the cavernous sinus.
Dural arteriovenous fistulas (DAVFs) are abnormal arteriovenous shunts, which develop in the dura mater, usually within or near the walls of a dural sinus and are most common in the cavernous and transverse or sigmoid sinuses4,6). Anatomically, the cavernous sinus (CS) is an extradurally located sinus, while other dural sinuses are located between two dural walls in the cranial cavity3,7). A variety of strategies for treatment of aggressive DAVF of the CS have been reported in the literature, including transvenous and/or transarterial embolization, radiation therapy, and surgery. Recent studies have demonstrated that Gamma Knife radiosurgery (GKRS) is a safe, less-invasive, and effective treatment for low-flow DAVF of the CS when used alone or in combination with other treatment modalities3,6,7,13,17). Here, the authors report a case of paradoxical exacerbation of symptoms resulting from obstruction of the venous outflow after GKRS for treatment of a DAVF of the CS.
A previously healthy 59-year-old female presented with progressive right proptosis, chemosis, and ocular pain for two weeks. She denied a history of craniofacial trauma. There were no abnormal findings, including cranial nerve palsy, on the neurological examination. Brain magnetic resonance images provided a clue indicating DAVF of the right CS without edematous cerebral lesions (Fig. 1). Cerebral catheter angiography showed a right-sided DAVF of the CS supplied by the dural branches of the internal and external carotid arteries bilaterally, corresponding to a Barrow Type C DAVF. Venous drainage was mainly retrograde through the enlarged ipsilateral superior and inferior ophthalmic veins. Cortical venous reflux was observed via the petrosal vein in the right posterior fossa (Fig. 2). For treatment, a transfemoral route via ipsilateral jugular vein and inferior petrosal sinus was attempted repeatedly, however, the microcatheter could not be passed through the tortuous inferior petrosal sinus. The patient refused further endovascular treatment or surgical exposure. Accordingly, the patient underwent GKRS at a marginal dose of 17 Gy on the prescription 50% isodose line and a maximal dose of 34 Gy with a volume of 611.2 mm3. No obvious change in her ocular symptoms was observed after GKRS.
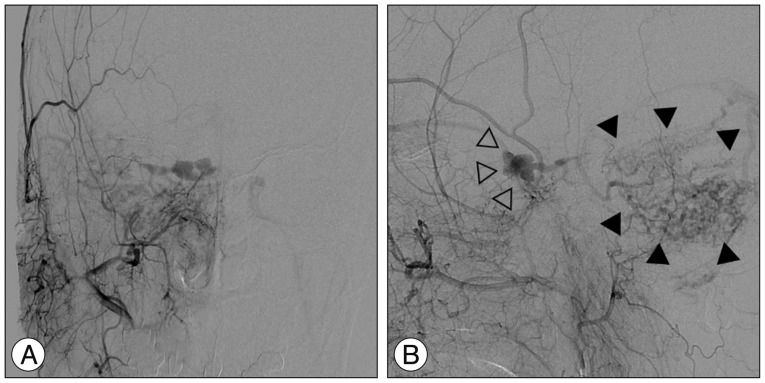
At one month after GKRS, she developed increasing ocular pain and occipital headache, and was readmitted to our hospital. She was not able to sleep less and less because headache was getting worse. However, no obvious change in her ocular symptoms such as proptosis and chemosis was observed, compared with the time of discharge. No abnormal findings were observed during neurological examination. T2-weighted magnetic resonance images showed a diffuse hyperintensity of the right cerebellar hemisphere (Fig. 3A). Diffusion weighted imaging showed a normal signal (Fig. 3B). Urgent cerebral angiography showed partial obliteration of the fistula and loss of drainage via the right superior and inferior ophthalmic veins. Severe congestion with slow flow was observed around the right cerebellar hemisphere (Fig. 4). Therefore, prompt transarterial embolization was attempted with the aim of symptom palliation. Under local anesthesia, a microcatheter was superselectively navigated into the feeders arising from both external carotid arteries via the middle meningeal artery, ascending pharyngeal artery, and internal maxillary artery. We used gelform mixed with contrast media and fibered coils for embolization. Immediate postembolization angiograms showed total occlusion of the external carotid artery feeders and disappearance of the cortical venous reflux in the right cerebellar hemisphere (Fig. 5), while the fistula supplied from the left internal carotid artery remained minimally. The patient showed rapid resolution of the pain and gradual improvement of other ocular symptoms. Conservative management, including manual compression of both carotid arteries, was continued following this endovascular procedure. No symptoms suggestive of recurrence developed during the clinical follow-up period of seven months.
DAVFs of the CS are somewhat different from other DAVFs, which drain into the transverse, sigmoid, or superior sagittal sinus. Cavernous sinus DAVFs are associated with a low risk of intracranial hemorrhage, unless cortical venous drainage is present, which occurs less frequently7). The clinical presentation is closely related to the pattern of venous drainage of DAVFs. Spontaneous remissions sometimes occur and are regarded as benign.
A variety of strategies for the treatment of aggressive DAVFs of the CS have been reported in the literature, including transvenous and/or transarterial embolization, radiation therapy and surgery. GKRS is reported mainly for those DAVFs with a high treatment risk involving surgical excision or embolization, as well as for lesions that fail more traditional modalities or require further adjunctive therapy4,11). GKRS targets the arterial feeders of the DAVF's nidus to induce diminishing shunting and eventually obliteration. Although it is not entirely clear how GKRS achieves obliterations, one theory points to radiation-induced small muscle expansion, adventitial fibrosis, and an intimal response leading to small vessel occlusion11). Another potential theory is that an inflammatory process in the adjacent dura causes external pressure on small arteries, leading to reduced blood flow and thrombosis4).
DAVFs of the CS are known to show a much more rapid response to GKRS than DAVFs that drain to sinuses other than the CS. Clinical improvements after GKRS are known to occur as early as two months after the procedure1,12,13,16). As reported by the largest study of DAVFs treated by GKRS, published by Wu et al.17), symptom improvement occurred as early as six weeks after GKRS. The angiography-documented obliteration rate after GKRS as a first-line treatment was 75% in the study reported by Wu et al.17) and 91% in the study by Barcia-Salorio et al.1). In a study of 20 cases of DAVFs of the CS, Pollock et al.15) reported a clinical improvement rate of 95% (19 of 20 patients) and an angiography-documented complete obliteration rate of 87% (13 of 15 patients).
According to other published papers on GKRS for treatment of DAVFs of the CS, administration of a high dose (margin dose >18 Gy) resulted in an occlusion rate of more than a 72% throughout variable follow-up periods1,4,8,14,15,17). Such a high radiation dose is very similar to that used elsewhere in GKRS for treatment of arteriovenous malformations5). In our case, when a high radiation dose was delivered to the delineated target, the optic apparatus, which is known to be sensitive to radiation, was exposed to less than 10 Gy. No radiation injuries were detected during our patient's follow-up period.
A major disadvantage is the necessary latency period until obliteration could be achieved with radiosurgery. Conversely, the treatment latency of radiosurgery could be an advantage in preventing the risk of venous hypertension and infarction involved with sudden complete obliteration by endovascular methods. Chiou et al.2) observed normalization of the reverse pulsatile flow in the superior ophthalmic vein at months after GKRS for DAVFs of the CS, indicating a gradual change of haemodynamics. Nevertheless, acute obstruction of the venous outflow at just one month after GKRS, like the current case, is a rare event. Lau et al.10) reported on a patient with worsening proptosis and ophthalmoplegia caused by transient superior ophthalmic vein thrombosis of a DAVF of the CS after GKRS.
The cavernous sinus normally receives drainage from the superior and inferior ophthalmic veins as well as superiorly from the sphenoparietal sinus, sylvian veins, and cortical veins. The cavernous sinus drains posteriorly through the inferior petrosal sinus and superior petrosal sinus to the jugular bulb, inferiorly through the pterygoid plexus via emissary veins, and contralaterally through the contralateral cavernous sinus9). In the current case, the right superior and inferior ophthalmic veins as the main venous outflow were occluded without diminishing shunting early after GKRS, resulting in serious cerebellar congestion due to drainage obstruction. It has been postulated that obstruction of the main venous outflow may be due to concurrent thrombus formation in the ophthalmic veins and in the anterior cavernous sinus, which depicts the mechanism of paradoxical worsening of symptoms before clinical improvement, after receiving GKRS.
Acknowledgements
This work was supported by clinical research grant from Pusan National University Hospital 2014.
References
1. Barcia-Salorio JL, Soler F, Barcia JA, Hernández G. Stereotactic radiosurgery for the treatment of low-flow carotid-cavernous fistulae : results in a series of 25 cases. Stereotact Funct Neurosurg. 1994; 63:266–270. PMID: 7624645.

2. Chiou HJ, Chou YH, Guo WY, Teng MM, Hsu CC, Tiu CM, et al. Verifying complete obliteration of carotid artery-cavernous sinus fistula : role of color Doppler ultrasonography. J Ultrasound Med. 1998; 17:289–295. PMID: 9586700.

3. Choi BS, Park JW, Kim JL, Kim SY, Park YS, Kwon HJ, et al. Treatment Strategy Based on Multimodal Management Outcome of Cavernous Sinus Dural Arteriovenous Fistula (CSDAVF). Neurointervention. 2011; 6:6–12. PMID: 22125741.

4. Dalyai RT, Ghobrial G, Chalouhi N, Dumont AS, Tjoumakaris S, Gonzalez LF, et al. Radiosurgery for dural arterio-venous fistulas : a review. Clin Neurol Neurosurg. 2013; 115:512–516. PMID: 23481896.
5. Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002; 63:347–354. PMID: 12142099.

6. Ghobrial GM, Marchan E, Nair AK, Dumont AS, Tjoumakaris SI, Gonzalez LF, et al. Dural arteriovenous fistulas : a review of the literature and a presentation of a single institution's experience. World Neurosurg. 2013; 80:94–102. PMID: 22381858.

7. Jung HH, Chang JH, Whang K, Pyen JS, Chang JW, Park YG. Gamma Knife surgery for low-flow cavernous sinus dural arteriovenous fistulas. J Neurosurg. 2010; 113(Suppl):21–27. PMID: 21121783.

8. Kida Y. Radiosurgery for dural arteriovenous fistula. Prog Neurol Surg. 2009; 22:38–44. PMID: 18948718.

9. Korkmazer B, Kocak B, Tureci E, Islak C, Kocer N, Kizilkilic O. Endovascular treatment of carotid cavernous sinus fistula : a systematic review. World J Radiol. 2013; 5:143–155. PMID: 23671750.

10. Lau LI, Wu HM, Wang AG, Yen MY, Hsu WM. Paradoxical worsening with superior ophthalmic vein thrombosis after gamma knife radiosurgery for dural arteriovenous fistula of cavernous sinus : a case report suggesting the mechanism of the phenomenon. Eye (Lond). 2006; 20:1426–1428. PMID: 16485013.

11. Loumiotis I, Lanzino G, Daniels D, Sheehan J, Link M. Radiosurgery for intracranial dural arteriovenous fistulas (DAVFs) : a review. Neurosurg Rev. 2011; 34:305–315. discussion 315. PMID: 21584687.

12. O'Leary S, Hodgson TJ, Coley SC, Kemeny AA, Radatz MW. Intracranial dural arteriovenous malformations : results of stereotactic radiosurgery in 17 patients. Clin Oncol (R Coll Radiol). 2002; 14:97–102. PMID: 12069135.
13. Onizuka M, Mori K, Takahashi N, Kawahara I, Hiu T, Toda K, et al. Gamma knife surgery for the treatment of spontaneous dural carotid-cavernous fistulas. Neurol Med Chir (Tokyo). 2003; 43:477–482. discussion 482-483. PMID: 14620198.

14. Pan HC, Sun MH, Yang DY, Wang YC, Lee SD, Chen WH, et al. Multidisciplinary treatment of cavernous sinus dural arteriovenous fistulae with radiosurgery and embolization. J Clin Neurosci. 2005; 12:744–749. PMID: 16169730.

15. Pollock BE, Nichols DA, Garrity JA, Gorman DA, Stafford SL. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999; 45:459–466. discussion 466-467. PMID: 10493367.

16. Söderman M, Edner G, Ericson K, Karlsson B, Rähn T, Ulfarsson E, et al. Gamma knife surgery for dural arteriovenous shunts : 25 years of experience. J Neurosurg. 2006; 104:867–875. PMID: 16776329.

17. Wu HM, Pan DH, Chung WY, Guo WY, Liu KD, Shiau CY, et al. Gamma Knife surgery for the management of intracranial dural arteriovenous fistulas. J Neurosurg. 105(Suppl):2006; 43–51. PMID: 18503329.

Fig. 1
A : Initial computed tomographic scan shows no abnormal finding. B : Contrast enhanced magnetic resonance image shows contrast leakage into the right cavernous sinus (arrow) suspicious for a dural arteriovenous fistula of the cavernous sinus. Right-sided exophthalmos is demonstrated.
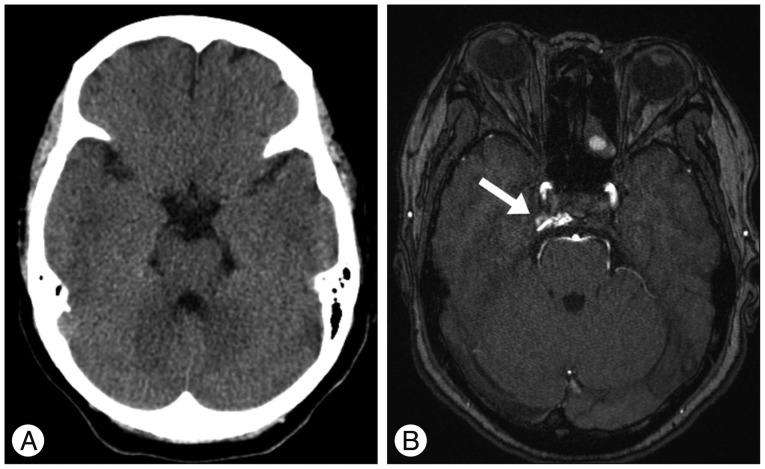
Fig. 2
Late arterial phase of the right external carotid artery (ECA) angiograms, anteroposterior (A) and lateral (B) projection, show a right-sided dural arteriovenous fistula of the cavernous sinus, which was supplied by multiple dural branches of the ECA with venous drainage into the right superior petrosal sinus (solid arrow), right superior ophthalmic vein (open arrow), and right inferior ophthalmic vein (open arrowheads). Cortical venous reflux (solid arrowheads) is seen in the right posterior fossa via the right petrosal vein.
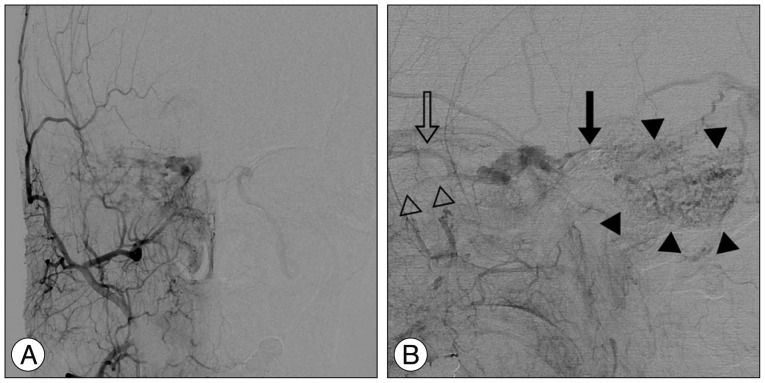
Fig. 3
A : T2-weighted MR image obtained on one month after Gamma Knife radiosurgery shows a diffuse hyperintensity of the right cerebellar hemisphere. B : Diffusion weighted image shows a normal signal.
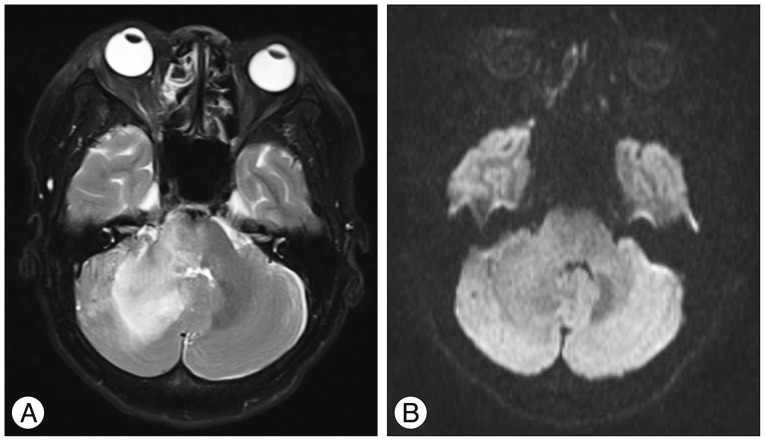
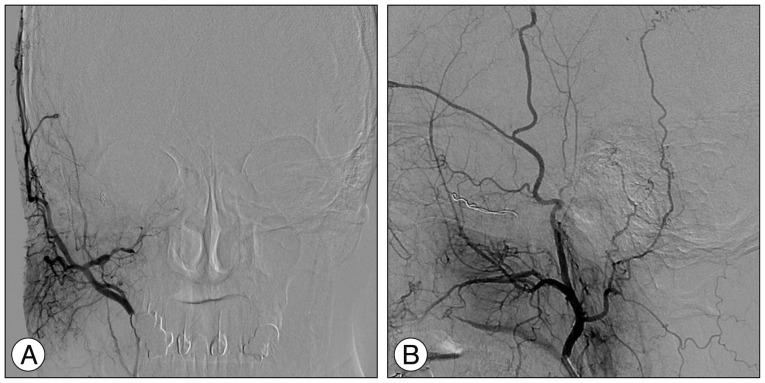
Fig. 4
Urgent catheter angiographies obtained one month after Gamma Knife radiosurgery, anteroposterior (A) and lateral (B) projection of the right external carotid artery injection, show partial obliteration of the fistula and loss of drainage via the right superior and inferior ophthalmic veins (open arrowheads) with severe congestion resulting in slow flow around the right cerebellar hemisphere (solid arrowheads).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download