Abstract
Aging process can be characterized as a spontaneous decrease of function in various organs with age. Muscle, as a big organ of human body, undergoes aging process presenting with loss of muscle mass, "sarcopenia". Recently, several working groups have tried to make consensus about sarcopenia for definition and diagnosis. Muscle mass is known to be closely related with bone, brain, fat, cardiovascular and metabolic systems. With increased understanding, clinical and basic researches about sarcopenia have been also increased rapidly from various areas of health science and technology. In this paper, the history and recent concepts of sarcopenia were reviewed and brief discussion of its prospect in the field of neurosurgery was done.
We are living in the aging society with rapid increase of the proportion of aged population. Accordingly, geriatric syndrome became an important medicosocial issue recently45). Muscle wasting is one of the main changes of aging human body, which was introduced by Rosenberg79,80) as the term sarcopenia in 1989. The loss of muscle mass is estimated about 8%/decade from 40 to 70 years of age, and then it becomes faster up to 15%/decade35). Sarcopenia has been widely known to be the decreased mass and strength of skeletal muscle by an aging process, but there are still debates in its definition67).
The European Union Geriatric Medicine Society (EUGMS) made a Sarcopenia Working Group, the European Working Group on Sarcopenia in Older People (EWGSOP), in 2009, and reported the European consensus on definition and diagnosis of sarcopenia in 201020). This European consensus seemed to trigger interest and attention for sarcopenia at many countries including Korea. The first annual meeting of Korean Sarcopenia Society was held at 2013. In 2014, the Asian consensus for sarcopenia was reported by the Asian Working Group for Sarcopenia (AWGS)14). There are several well-known operational definitions of sarcopenia based on skeletal muscle mass index (SMI)3,47,70,72), but the definition of EWGSOP combined both the quantitative factor (SMI) and qualitative factors (strength and function)20). The definitions are crucial for not only diagnosis but also treatment strategy of sarcopenia.
Sarcopenia is known to associate with various age related disorders, obesity15), metabolic syndrome83), cardiovascular disease83), osteoporosis23,58), and stroke86). It already became hot topic for clinical and basic researches in the various medical fields like endocrinology, cardiology, rehabilitation medicine, and orthopedics. Most of the researchers are convincing the sarcopenia will make a tremendous and multidirectional impact on medical care system in the near future. But, most of neurosurgeons do not seem to have interests in the muscle condition which may have effects on clinical courses of some neurosurgical diseases.
In this paper, up-to-date information of sarcopenia is introduced with review of literature. The relationship of sarcopenia with neurosurgical disorders and the roles of neurosurgeons are also discussed.
Sarcopenia can be characterized by progressive loss of SMI and muscle strength with increasing risk of physical disability, poor quality of life, and sometimes death22,34). There are several definitions based on muscle mass. The dual energy X-ray absorptiometry (DXA) and bioelectrical impedance analysis (BIA) can be used for assessing SMI, and magnetic resonance imaging (MRI) and computerized tomography (CT) for muscle cross-sectional area (CSA).
The first reported definition of sarcopenia is based on DXA3). DXA can measure the appendicular SMI (ASM) at the fat free muscle mass of arm and leg96). The definition of sarcopenia with DXA is the reduction of ASM/height2) less than or equal to two standard deviations (SD) comparing to the sex specific mean reference values of a young age (18-40 years) group3).
SMI measured by BIA can be used for definition of sarcopenia. Class I and II sarcopenia are defined as the SMI less than one and two SD comparing to that of the sex specific mean reference value of young age (18-39 years) group, respectively47).
CSA of muscle by MRI or CT is considered to be the most accurate data. The body weight (BW) corrected quadriceps (Qc) muscle CSA (Qc muscle CSA/BW) reflects thigh muscle mass71). Qc Sarcopenia can be defined as the BW corrected Qc muscle CSA more than 1 SD below the mean reference value of younger age (<60 years) group72).
However, the muscle mass itself is not enough for diagnosis of sarcopenia because it is not correlated with its strength34,46). The EWGSOP definition includes SMI and muscle function, requiring
2 or 3 criteria among three diagnostic criteria (low muscle mass, low muscle strength, and low physical performance) for diagnosis
of sarcopenia20). The tools for assessing sarcopenia recommended
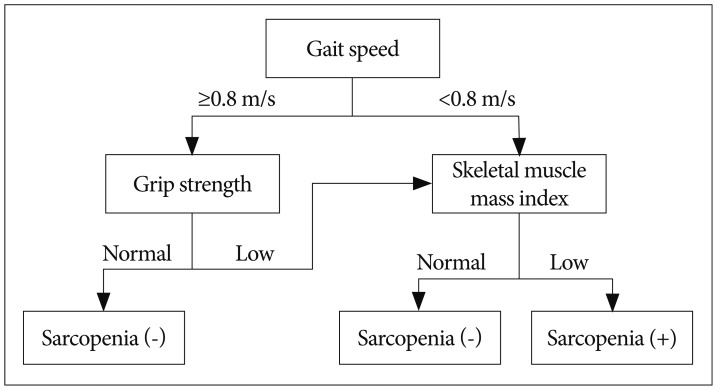
by EWGSOP are CT, MRI, and DXA for muscle mass, grip strength for muscle strength53), and short physical performance battery (SPPB) or gait speed for physical performance36,37,100). SPPB score is total points of three tests, balance, gait speed, and chair stand, scoring between 0-4 for each test with maximum score of 1236). Cut-off points for the three components differ according to the technique used for assessment. The cut-off points for SMI measured with DXA are 7.26 kg/m2 for men and 5.5 kg/m2 for women by Baumgartner method3), 7.23 kg/m2 for men and 5.67 kg/m2 for women by Newman method70), and 7.25 kg/m2 for men and 5.67 kg/m2 for women by Delmonico method22). Cut-off points for the muscle strength are <30 kg for men and <20 kg for women by Laurentani method53) or different muscle strength levels according to BMI introduced by Fried et al.31). Cut-off point for the physical performance is SPPB score ≤8 or gait speed <0.8 m/s37,100). The EWGSOP-suggested diagnostic algorithm is started with the measurement of gait speed for the patient older than 65 years (Fig. 1); 1) With the gait speed >0.8 m/s, no sarcopenia is defined as the grip strength is normal, but SMI should be measured as a final assessment for diagnosis of sarcopenia when the grip strength is subnormal, 2) With the gait speed ≤0.8 m/s, SMI is the next step as a final assessment for diagnosis of sarcopenia.
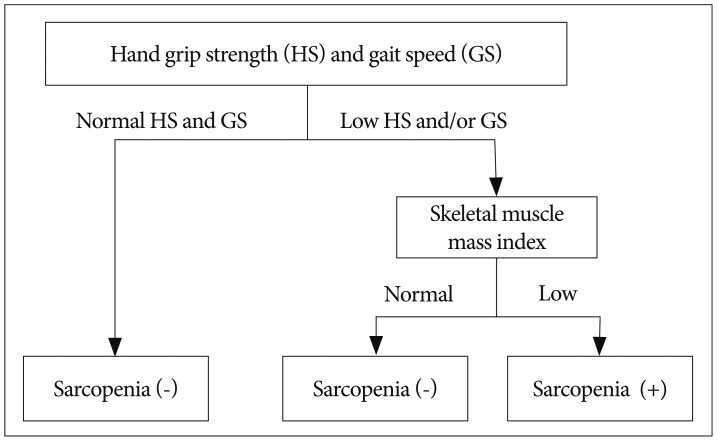
The AWGS recommended cut-off points are SMI <7.0 kg/m2 for men and <5.4 kg/m2 for women by DXA or <7.0 kg/m2 for men and <5.7 kg/m2 for women by BIA, handgrip strength <26 kg for men and <18 kg for women, and gait speed ≤0.8 m/s14). The diagnostic algorithm of AWGS for sarcopenia starts with checking both handgrip strength and gait speed (Fig. 2). When the handgrip strength and/or gait speed less than cut-off points, then muscle mass is measured for diagnosis of sarcopenia14).
Baumgartner et al.3) reported the prevalence of sarcopenia about 13-24% in the older patients (65-70 years) and increased up to 50% in the patients over 80 years of age, and it was higher in male group. Another study reported the prevalence of sarcopenia varies 6-15% in the subjects over 65 years of age with higher prevalence in male group63). Sarcopenia also seemed to increase the rate of disability about 4.1 times and 3.6 times more in male and female groups comparing to the non-sarcopenic patients3).
Classically, skeletal muscle fibers are divided into two types, type 1 and 269). Type 1 fibers are known to be white muscle, fatigue resistant and slow fibers specialized for phasic contraction (e.g., marathon)38). Type 2 fibers are red muscle, faster and stronger than Type 1 fibers specialized for continuous contraction (e.g., weight lifting)38). The fatigue resistance of slow fibers related primarily with the use of oxidative metabolism by Type 1 fibers whereas glycolytic metabolism by Type 2 fibers77). The Type 2 fibers are known to be the primary target for age related muscle atrophy28). Varieties of factors, neurodegeneration, endocrinological environment, inflammatory dysfunction, and obesity have been proposed as the causes of sarcopenia.
The age related decrease in the number of motor neurons in spinal cord52) and peripheral nerve fibers and synaptic vesicles in neuromuscular junction24) had been reported to reduce SMI.
Hormonal factors for maintaining muscular protein metabolism, growth hormone, insulin-like growth factor-1, corticosteroids, androgens, vitamin D, and insulin, are known to decrease with age influencing on SMI negatively25,50,84,85,92,103).
Some pro-inflammatory factors, interleukin-6 (IL-6), plasminogen activator inhibitor 1, and C-reactive protein, were reported to increase in plasma in the elderly people, which is closely related with the age related increase of body fat mass (adipokines)13,42). The low level inflammation by the increased pro-inflammatory factors with wasting of protein and energy seems to have effect on the sarcopenia in the elderly with obesity (sarcopenic obesity)49,87,88). Myokines, myogenic hormone like factors including IL-6, IL-8, IL-15, brain-derived neurotropic factor, and fibroblast growth factor-21, are known to increase during exercise showing protective effect in contrast to the harmful effect of adipokines for skeletal muscle75,76,95). IL-6 and IL-15 secreted from muscles are able to induce lipolysis of fat, so called "muscle-fat crosstalk"76).
Skeletal muscle can be regenerated through activation of satellite cells, undifferentiated mononuclear myogenic cells in skeletal muscles9,61). The satellite cells are known to be the adult stem cells of muscle, which tend to decrease in number and function with age40,74). According to previous reports, aged satellite cells show tendency to differentiate into adipose and fibrotic tissues7,90). Myostatin, a member of transforming growth factor-beta family, can inhibit muscle growth62). Myostatin is thought to inhibit satellite cells proliferation according to previous studies about the relationship between myostatin level and satellite cell population in skeletal muscles12,98). Activin A is known to inhibit muscle growth through binding with type II activin receptor55). Both myostatin and activin A were also reported to inhibit bone mineralization by osteoblast26,27). In contrast to the negative effect of myostatin and activin A on muscle and bone, follistatin can play a role of enhancing muscle growth and bone mineralization by antagonizing both myostatin and activin A26,33). Myostatin and activin A have been observed to increase with age, whereas follistatin did not show correlation with age2,56). The activin A-myostatin-follistatin system means the interactions among the three factors and seems to play an important role in both muscle growth and bone mineralization during aging process6). The terms "muscle-bone interaction" and "muscle-bone cross-talk" are hence proposed according to the concept that skeletal muscle and bone continuously affect and modulate each other's biological properties not only biomechanically but also endocrinologically17,39).
Sarcopenic obesity is the imbalance between muscle and fat mass, a combination of decreased SMI and increased visceral fat, in elderly people104). It is closely associated with reduced muscle strength and physical activity, metabolic disorders, and sometimes age related mortality18,82,88,104). In sarcopenic obesity, the decrease physical activity by muscle atrophy results in accumulation of body fat which will increase the levels of pro-inflammatory factors leading to muscle atrophy81,87). In addition, the muscle loss can induce metabolic disorders related with insulin resistance because skeletal muscle is the largest organ response to insulin51,104).
It seems that there are fragility fractures in the patients without osteoporosis according to BMD study or without high fracture risk calculated with World Health Organization fracture assessment tool94,97). The increased fracture risk with age seems to be greater than the age related reduction of BMD48). Recent studies revealed that patients with both osteoporosis and sarcopenia (sarco-osteoprosis) showed greater risk for fracture4,19). Buehring et al.8) reported the prevalences of sarcopenia, osteopenia, and sarco-osteopenia were about 15%, 17%, and 5% of the population with age ≥60 years.
Osteosarcopenic obesity was introduced by Ormsbee et al.73) in 2014 to define the individuals with obesity, low BMD, and sarcopenia. They said the obesity in addition to the pre-existing sarco-osteoporosis would exacerbate the metabolic disorders leading to poor physical function and quality of life. But there are still debates without conclusive diagnostic criteria for osteosarcopenic obesity even though it is one of the top emerging significant public health issues.
Fried et al.31) first proposed definition for frailty in 2001 as an age related syndrome including weight loss, exhaustion, weakness of grip strength, slow walking speed, and low physical activity. Recent consensus for frailty definition is "a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance and reduced physiologic function that increases an individual's vulnerability for developing increased dependency and/or death", which means sarcopenia combined with fatigue caused by many pathologic conditions68,91).
In 2008, Evans et al.29) introduced new definition of the cachexia, according to the agreement at the cachexia consensus conference, as a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass. They proposed a multidimensional diagnostic assessment for cachexia including weight loss >5% for one year or BMI <20 with any three components of the followings : fatigue, anorexia, decreased muscle strength, anemia (hemoglobin <12 g/dL), hypoalbuminemia (<3.2 g/dL), or elevated inflammatory markers like IL-6 and C-reactive protein29). The major characteristics of cachexia comparing to sarcopenia are comorbid pathologic conditions (cancer, chronic heart failure, chronic obstructive pulmonary disease, etc.), decreased fat mass, systemic inflammation, increased protein wasting, increased resting energy expenditure, and anorexia89).
Most of studies for exercise therapy focused on progressive resistance training (PRT) to improve muscle strength and function rather than aerobic exercise. Yarasheski et al.101) recommended PRT for elderly population (76-92 years), 3 days/week for 3 months. They reported increase of whole body muscle mass about 2.2 kg for men and 1.0 kg for women. Gate speed and muscle strength were also known to be improved with PRT31,59).
Sarcopenic elderly individuals are recommended to intake protein of 1.0-1.5 g/kg/day which is about 15-20% of daily caloric intake5,66). Supplementary Vitamin D (700 IU/day) is recommended for the patients with vitamin D level <100 nmol/L5,66). Creatine monohydrate is also known to helpful for energy storage by increasing availability of phosphocreatine10). Chrusch et al.16) reported creatine supplement with PRT increased muscle mass as well as improved muscle strength.
Motor paralysis induces early changes in muscle tissue by decreased motor nerve signals and reduction of number of motor units1). During chronic phase after stroke, disuse and spasticity can result in muscle atrophy and muscle type shift11). The distribution of muscle fiber types also changes after stroke from type 1 slow twitch muscle fibers to type 2 fast twitch muscle fibers, which is an inverse direction of age related muscle fiber type shift21). The muscle type shift after stroke was reported to affect clinical result negatively by aggravating gait disturbance with decreased walking speed of paralyzed leg21). Malnutrition is a common problem affecting clinical outcome of stroke patients, and it also affect the development of sarcopenia66,102).
Despite the negative influences of sarcopenia on the prognosis of stroke, guidelines for treatment, prevention, and rehabilitation of stroke patients do not indicate any consideration about stroke related sarcopenia32,77). Sarcopenia seems to be overlooked during management of patients after stroke.
Muscles consist of major portion of spine, but spine surgeons seem to not well understand the nature of muscles around spine comparing to that of bone, joint, or spinal nerves. Osteoporosis is one of the leading problems of spine in the elderly population, and is known to be closely related with sarcopenia through muscle-bone cross-talk17,39). There are strong evidences that muscular atrophy and fat infiltration are the representative age related degenerative changes of muscles54,57,93). Both the atrophy and fatty change of paraspinal muscles are also known to be associated with functional disorders and chronic back pain44,60,64,65). Degenerative lumbar spinal deformities including flat back syndrome and degenerative lumbar kyphosis are also reported to associate with paraspinal muscular atrophy and fatty change43,54).
In spite of many evidences for the relationship between paraspinal muscular atrophy and spinal disorders, there is still lack of concept to link the spinal problems with sarcopenia. The main reason of this mismatch may come from the definition of sarcopenia as a systemic disorder.
Muscles seem to have been underestimated in relation to the neurosurgical disorders. Even though sarcopenia is not yet listed in the 2014 release of international classification of disease, it must be an essential part of clinical practice and research by neurosurgeons in the near future.
References
1. Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, Hyodo A, et al. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006; 250:27–32. PMID: 16904126.

2. Baccarelli A, Morpurgo PS, Corsi A, Vaghi I, Fanelli M, Cremonesi G, et al. Activin A serum levels and aging of the pituitary-gonadal axis : a cross-sectional study in middle-aged and elderly healthy subjects. Exp Gerontol. 2001; 36:1403–1412. PMID: 11602213.

3. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998; 147:755–763. PMID: 9554417.

4. Binkley N, Buehring B. Beyond FRAX : it's time to consider "sarco-osteopenia". J Clin Densitom. 2009; 12:413–416. PMID: 19733110.
5. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D : a meta-analysis of randomised controlled trials. BMJ. 2009; 339:b3692. PMID: 19797342.
6. Bowser M, Herberg S, Arounleut P, Shi X, Fulzele S, Hill WD, et al. Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells. Exp Gerontol. 2013; 48:290–297. PMID: 23178301.

7. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007; 317:807–810. PMID: 17690295.

8. Buehring B, Krueger D, Binkley N. Effect of including historical height and radius BMD measurement on sarco-osteoporosis prevalence. J Cachexia Sarcopenia Muscle. 2013; 4:47–54. PMID: 22872366.

9. Campion DR, Richardson RL, Reagan JO, Kraeling RR. Changes in the satellite cell population during postnatal growth of pig skeletal muscle. J Anim Sci. 1981; 52:1014–1018. PMID: 7240043.

10. Candow DG, Chilibeck PD. Effect of creatine supplementation during resistance training on muscle accretion in the elderly. J Nutr Health Aging. 2007; 11:185–188. PMID: 17435961.
11. Carda S, Cisari C, Invernizzi M. Sarcopenia or muscle modifications in neurologic diseases : a lexical or patophysiological difference? Eur J Phys Rehabil Med. 2013; 49:119–130. PMID: 23575206.
12. Carlson CJ, Booth FW, Gordon SE. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol. 1999; 277(2 Pt 2):R601–R606. PMID: 10444569.
13. Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005; 82:428–434. PMID: 16087989.

14. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia : consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc. 2014; 15:95–101. PMID: 24461239.

15. Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab (Seoul). 2013; 28:86–89. PMID: 24396659.

16. Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001; 33:2111–2117. PMID: 11740307.

17. Cianferotti L, Brandi ML. Muscle-bone interactions : basic and clinical aspects. Endocrine. 2014; 45:165–177. PMID: 23990248.
18. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008; 63:829–834. PMID: 18772470.

19. Coin A, Perissinotto E, Enzi G, Zamboni M, Inelmen EM, Frigo AC, et al. Predictors of low bone mineral density in the elderly : the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008; 62:802–809. PMID: 17637603.

20. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia : European consensus on definition and diagnosis : Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39:412–423. PMID: 20392703.

21. De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004; 30:209–215. PMID: 15266637.

22. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007; 55:769–774. PMID: 17493199.

23. DiGirolamo DJ, Kiel DP, Esser KA. Bone and skeletal muscle : neighbors with close ties. J Bone Miner Res. 2013; 28:1509–1518. PMID: 23630111.
24. Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit : a brief review. Can J Appl Physiol. 1993; 18:331–358. PMID: 8275048.
25. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985). 2008; 104:1452–1461. PMID: 18323467.

26. Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, et al. The activin A-follistatin system : potent regulator of human extracellular matrix mineralization. FASEB J. 2007; 21:2949–2960. PMID: 17449718.

27. Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010; 10:56–63. PMID: 20190380.
28. Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993; 123(2 Suppl):465–468. PMID: 8429405.

29. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia : a new definition. Clin Nutr. 2008; 27:793–799. PMID: 18718696.
30. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990; 263:3029–3034. PMID: 2342214.

31. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults : evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156. PMID: 11253156.
32. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack : a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011; 42:227–276. PMID: 20966421.

33. Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009; 297:E157–E164. PMID: 19435857.

34. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults : the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006; 61:1059–1064. PMID: 17077199.

36. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability : consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000; 55:M221–M231. PMID: 10811152.
37. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function : association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49:M85–M94. PMID: 8126356.
38. Guth L, Samaha FJ. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969; 25:138–152. PMID: 4241609.

39. Hamrick MW. The skeletal muscle secretome : an emerging player in muscle-bone crosstalk. Bonekey Rep. 2012; 1:60. PMID: 23951457.
40. Hikida RS. Aging changes in satellite cells and their functions. Curr Aging Sci. 2011; 4:279–297. PMID: 21529324.

41. Hoffman JR, Kraemer WJ, Bhasin S, Storer T, Ratamess NA, Haff GG, et al. Position stand on androgen and human growth hormone use. J Strength Cond Res. 2009; 23(5 Suppl):S1–S59. PMID: 19620932.

42. Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007; 86:633–638. PMID: 17823427.

43. Hyun SJ, Bae CW, Lee SH, Rhim SC. Fatty degeneration of paraspinal muscle in patients with the degenerative lumbar kyphosis : a new evaluation method of quantitative digital analysis using MRI and CT scan. J Spinal Disord Tech. 2013; [Epub ahead of print].
44. Hyun SJ, Kim YB, Kim YS, Park SW, Nam TK, Hong HJ, et al. Postoperative changes in paraspinal muscle volume : comparison between paramedian interfascial and midline approaches for lumbar fusion. J Korean Med Sci. 2007; 22:646–651. PMID: 17728503.

45. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes : clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007; 55:780–791. PMID: 17493201.

46. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004; 159:413–421. PMID: 14769646.

47. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002; 50:889–896. PMID: 12028177.

48. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009; 4:e7038. PMID: 19753111.

49. Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status : the Korean Sarcopenic Obesity Study. Clin Endocrinol (Oxf). 2013; 78:525–532. PMID: 22563924.

50. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes : the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010; 33:1497–1499. PMID: 20413515.

51. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults : the Korean sarcopenic obesity study. Int J Obes (Lond). 2009; 33:885–892. PMID: 19564878.

52. Kullberg S, Ramírez-León V, Johnson H, Ulfhake B. Decreased axosomatic input to motoneurons and astrogliosis in the spinal cord of aged rats. J Gerontol A Biol Sci Med Sci. 1998; 53:B369–B379. PMID: 9754135.

53. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility : an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003; 95:1851–1860. PMID: 14555665.

54. Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis : comparison with the normal controls. Spine (Phila Pa 1976). 2008; 33:318–325. PMID: 18303466.

55. Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005; 102:18117–18122. PMID: 16330774.

56. Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008; 11:163B–175B. PMID: 18240972.

57. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995; 50(Spec No):11–16. PMID: 7493202.
58. Lynch NA, Ryan AS, Evans J, Katzel LI, Goldberg AP. Older elite football players have reduced cardiac and osteoporosis risk factors. Med Sci Sports Exerc. 2007; 39:1124–2230. PMID: 17596780.

59. Mangione KK, Miller AH, Naughton IV. Cochrane review : improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010; 90:1711–1715. PMID: 21123213.

60. Mannion AF, Taimela S, Müntener M, Dvorak J. Active therapy for chronic low back pain part 1 Effects on back muscle activation, fatigability, and strength. Spine (Phila Pa 1976). 2001; 26:897–908. PMID: 11317112.
61. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961; 9:493–495. PMID: 13768451.

62. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997; 387:83–90. PMID: 9139826.

63. Melton LJ 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000; 48:625–630. PMID: 10855597.

64. Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers : quantification with MR spectroscopy. Radiology. 2006; 240:786–792. PMID: 16926328.

65. Min SH, Kim MH, Seo JB, Lee JY, Lee DH. The quantitative analysis of back muscle degeneration after posterior lumbar fusion : comparison of minimally invasive and conventional open surgery. Asian Spine J. 2009; 3:89–95. PMID: 20404953.

66. Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010; 11:391–396. PMID: 20627179.

67. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001; 137:231–243. PMID: 11283518.

68. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus : a call to action. J Am Med Dir Assoc. 2013; 14:392–397. PMID: 23764209.
70. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia : alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003; 51:1602–1609. PMID: 14687390.

71. Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010; 212:327–332. PMID: 20554280.

72. Ochi M, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr Gerontol Int. 2010; 10:233–243. PMID: 20199590.

73. Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, et al. Osteosarcopenic obesity : the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle. 2014; [Epub ahead of print].
74. Pannérec A, Marazzi G, Sassoon D. Stem cells in the hood : the skeletal muscle niche. Trends Mol Med. 2012; 18:599–606. PMID: 22877884.
75. Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle fat cross talk. J Physiol. 2009; 587(Pt 23):5559–5568. PMID: 19752112.

76. Pedersen BK, Edward F. Adolph distinguished lecture : muscle as an endocrine organ : IL-6 and other myokines. J Appl Physiol (1985). 2009; 107:1006–1014. PMID: 19696361.

77. Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972; 11:2627–2633. PMID: 4261555.

78. Quinn TJ, Paolucci S, Sunnerhagen KS, Sivenius J, Walker MF, Toni D, et al. Evidence-based stroke rehabilitation : an expanded guidance document from the european stroke organisation (ESO) guidelines for management of ischaemic stroke and transient ischaemic attack 2008. J Rehabil Med. 2009; 41:99–111. PMID: 19225703.

79. Rosenberg IH. Sarcopenia : origins and clinical relevance. Clin Geriatr Med. 2011; 27:337–339. PMID: 21824550.
80. Rosenberg IH. Summary comments : epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989; 50:1231–1233.
81. Roubenoff R. Catabolism of aging : is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003; 6:295–299. PMID: 12690262.
82. Roubenoff R. Sarcopenic obesity : the confluence of two epidemics. Obes Res. 2004; 12:887–888. PMID: 15229325.
83. Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women : relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999; 23:126–132. PMID: 10078845.

84. Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008; 294:E961–E968. PMID: 18349113.

85. Sayer AA, Syddall HE, Dennison EM, Martin HJ, Phillips DI, Cooper C, et al. Grip strength and the metabolic syndrome : findings from the Hertfordshire Cohort Study. QJM. 2007; 100:707–713. PMID: 17951315.

86. Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced Sarcopenia : muscle wasting and disability after stroke. Int J Cardiol. 2013; 170:89–94. PMID: 24231058.
87. Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985). 2007; 102:919–925. PMID: 17095641.

88. Stenholm S, Rantanen T, HeliÖvaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008; 56:462–469. PMID: 18179481.

89. Tan BH, Fearon KC. Cachexia : prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008; 11:400–407. PMID: 18541999.
90. Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002; 123:649–661. PMID: 11850028.

91. Theou O, Jones GR, Overend TJ, Kloseck M, Vandervoort AA. An exploration of the association between frailty and muscle fatigue. Appl Physiol Nutr Metab. 2008; 33:651–665. PMID: 18641707.

92. Visser M, Deeg DJ, Lips P. Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia) : the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003; 88:5766–5772. PMID: 14671166.

93. Vlassopoulos A, Combet E, Lean ME. Changing distributions of body size and adiposity with age. Int J Obes (Lond). 2014; 38:857–864. PMID: 24247373.

94. Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005; 90:2787–2793. PMID: 15728213.

95. Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009; 73:13–18. PMID: 19043226.

96. Wang ZM, Visser M, Ma R, Baumgartner RN, Kotler D, Gallagher D, et al. Skeletal muscle mass : evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol (1985). 1996; 80:824–831. PMID: 8964743.

97. Watts NB. The Fracture Risk Assessment Tool (FRAX®) : applications in clinical practice. J Womens Health Larchmt. 2011; 20:525–531. PMID: 21438699.

98. Wehling M, Cai B, Tidball JG. Modulation of myostatin expression during modified muscle use. FASEB J. 2000; 14:103–110. PMID: 10627285.

99. Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003; 58:618–625. PMID: 12865477.

100. Working Group on Functional Outcome Measures for Clinical Trials. Functional outcomes for clinical trials in frail older persons : time to be moving. J Gerontol A Biol Sci Med Sci. 2008; 63:160–164. PMID: 18314451.
101. Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/= 76 yr old. Am J Physiol. 1999; 277(1 Pt 1):E118–E125. PMID: 10409135.
102. Yoo SH, Kim JS, Kwon SU, Yun SC, Koh JY, Kang DW. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. 2008; 65:39–43. PMID: 18195138.

103. Zadik Z, Chalew SA, McCarter RJ Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985; 60:513–516. PMID: 3972964.

104. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity : a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008; 18:388–395. PMID: 18395429.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download