Abstract
We report a case of cervicomedullary compression by an anomalous vertebral artery treated using microsurgical decompression with intraoperative monitoring. A 68-year-old woman presented with posterior neck pain and gait disturbance. MRI revealed multiple abnormalities, including an anomalous vertebral artery that compressed the spinal cord at the cervicomedullary junction. Suboccipital craniectomy with C1 laminectomy was performed. The spinal cord was found to be compressed by the vertebral arteries, which were retracted dorsolaterally. At that time, the somatosensory evoked potential (SSEP) changed. After release of the vertebral artery, the SSEP signal normalized instantly. The vertebral artery was then lifted gently and anchored to the dura. There was no other procedural complication. The patient's symptoms improved. This case demonstrates that intraoperative monitoring may be useful for preventing procedural complications during spinal cord microsurgical decompression.
Cervicomedullary compression caused by various abnormalities in bony and soft tissue is a common and well-characterized condition1,6,7). In contrast, cervicomedullary compression due to an anomalous change in the vertebral artery (VA), as in dolichoectasia, is a relatively rare condition. Intracranial arterial dolichoectasia is defined as an increase in the length and diameter of the intracranial arteries, and the vertebrobasilar system is the most commonly affected segment17,22). Dolichoectatic changes in the VA without involvement of the basilar artery are quite rare and can cause a pulsatile mass effect in the surrounding areas. For example, VA anomalies have been previously reported to cause a variety of symptoms, including neck and arm pain, occipital neuralgia, torticollis, myelopathy, drop attack, radiculopathy, pyramidal tract signs, and cranial nerve defects4,5,8,9,11,12,13,15,16,20,23,24,25,26). The few reported cases of this condition showed improvement in symptoms following microsurgical decompression2,4,5,15,21,23,24,25,26). However, adhesion or indentation of the VA with the low cranial nerve, spinal cord, and rootlet is common in dolichoectasia. Therefore, the potential for intraoperative complications due to damage to these adjacent structures during decompression should be carefully considered. In this case report, we show that the use of intraoperative monitoring (IOM) of somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP) aided in checking for potential nerve root or spinal cord damage during microvascular decompression in a case of cervicomedullary compression caused by an anomalous VA.
A 68-year-old woman had a 10-year history of posterior neck pain and a 3-year history of gait disturbance. She also complained of quadriparesis. Her symptoms worsened gradually. A neurological examination revealed normal muscle strength in all extremities, but her alternating motion rate was below normal. She had marked impairment in vibration and joint position senses in all four extremities. However, no abnormal findings were observed upon electromyography. Her pathological reflexes were not checked. MRI revealed multiple abnormalities, including an anomalous tortuous VA compressing the dorsal portion of the spinal cord bilaterally at the cervicomedullary junction, spinal cord signal changes, and atrophy (Fig. 1). CT angiography revealed that both the VAs were elongated and enlarged (Fig. 2). No congenital anomalies of the posterior fossa or any degenerative changes in the spinal column were observed.
IOM of SSEP and MEP using an intraoperative monitoring system (ISIS IOM system, inomed Medizintechnik GmbH, Teningen, Germany) was initiated under general anesthesia. After making a midline incision, suboccipital craniectomy with C1 laminectomy was performed. In order to avoid the risk of destabilizing the cervical vertebrae, C2 laminectomy was not performed. The muscles inserting into C2 were preserved. To protect the extradural VA, the back muscle was very carefully stripped using Doppler monitoring. Next, the dura was opened to form a Y shape revealing the tortuous VAs, which appeared somewhat sclerotic. The VAs were intertwined and they had formed severe adhesions with the C2 rootlet and spinal cord. We also found an indentation of the VAs with the spinal cord, and VA pulsations compressed the spinal cord (Fig. 3).
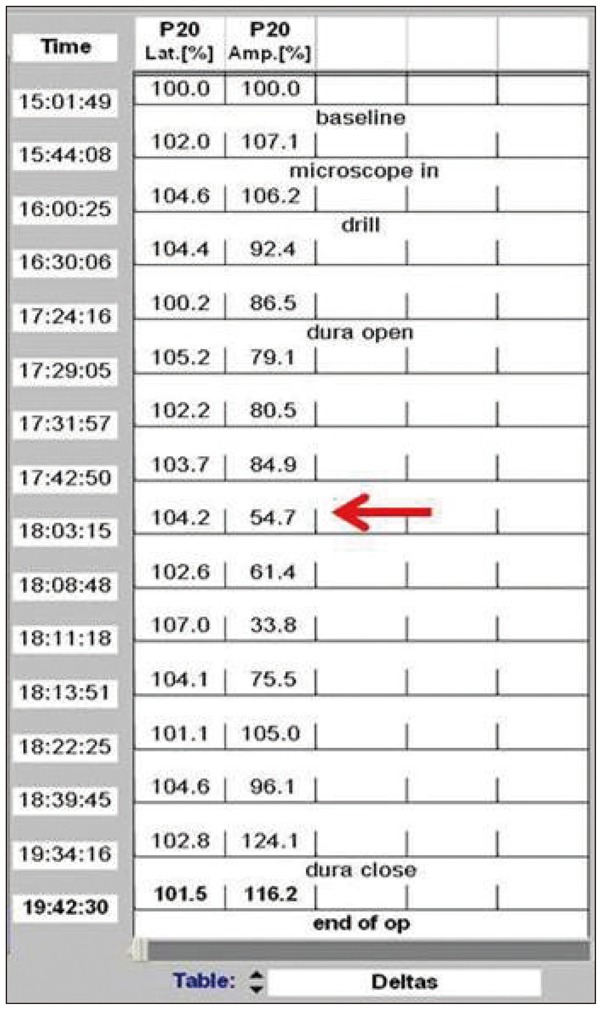
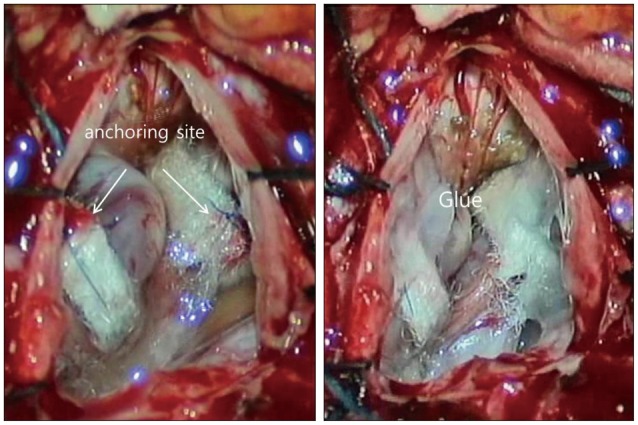
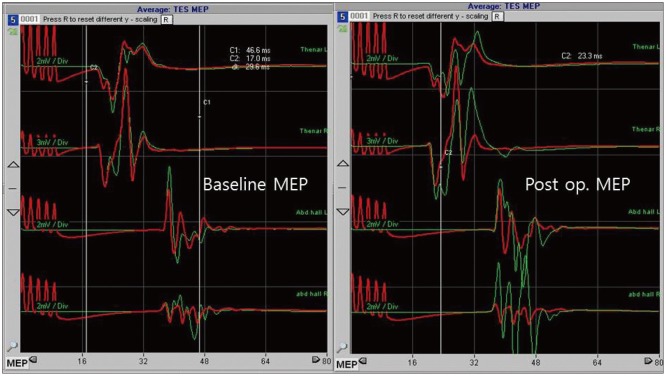
In order to decompress the spinal cord, the VAs were dissected and retracted dorsolaterally. At this time, the SSEP signal of the right tibialis anterior muscle changed, with the amplitude dropping to below 50% of baseline (Fig. 4). The VAs were, therefore, released. Instantly, the SSEPs signal normalized. Subsequently, the VAs were dissected with extreme care and then lifted gently without causing any changes in SSEP. In comparison with the initial dissection, the latter procedure was performed very slowly with no resistance. Teflon sponges were passed around the arteries on each side to create a sling. The sponges were then anchored to the dura using silk sutures. The entire area was bonded with surgical glue (Fig. 5). Duraplasty was performed using artificial dura. During the surgery, no changes in MEP were detected. An increase in MEP amplitude compared to baseline was, however, observed after dural closure (Fig. 6). Following meticulous bleeding control, the wound was closed layer-by-layer, and there were no other procedural complications.
Postoperatively, the patient experienced marked improvement in her symptoms. The day after surgery, her posterior neck pain improved. Her gait disturbance improved gradually. Six months after the surgery, the patient complained of no symptoms except numbness in the occipital area.
Cervicomedullary compression caused by an anomalous change in the VA is a rare condition. Many patients with condition have been successfully treated by microvascular decompression using various materials2,4,5,15,21,23,24,25,26). However, two major risks are associated with this treatment option. The first is spinal cord damage during VA dissection, and the second is instability of the spinal column following surgery.
Given the complex structure of the cervicomedullary junction, vascular compromise and resulting damage to the spine during surgery is a major concern. The main arteries supplying blood to medulla and spinal cord are the posterior inferior cerebellar artery, anterior spinal artery, and posterior spinal artery. Surgical manipulation of the VA does not affect these main arteries supplying blood to the spinal cord, but it may affect microcirculation in the spinal cord. It is difficult to detect damage to microvessels using standard intraoperative vascular monitoring techniques, such as intraoperative indocyanine green videoangiography or Doppler sonography. Therefore, we performed continuous IOM of SSEP and MEP to monitor potential spinal cord damage.
In the field of spinal surgery, intraoperative monitoring of SSEP was first introduced to avoid undesirable neurological complications14). However, SSEP is a measure of sensory nerve function that is mainly localized in the dorsal column. Therefore, SSEP cannot indicate any anterior spinal column damage, including damage to the corticospinal and motor tracts27). As a result, surgeons have questioned the reliability of SSEP for monitoring damage throughout the spinal cord. Improved technology has enabled intraoperative MEP monitoring of the motor component of the spinal cord. Thus, both MEP and SSEP can now be monitored during spinal surgery, increasing the sensitivity for detecting spinal cord damage3,10).
The patient described here underwent microsurgical decompression for cervicomedullary compression caused by anomalous VAs. The VAs directly adhered to and compressed the dorsal column. However, the patient was at risk of not only damage to the dorsal column, but also intraoperative damage to the motor tract. During decompression, we monitored both the MEP and SSEP signals. In standard surgical decompression for VAs that compress the spinal cord and entangle rootlets, the VAs are retracted dorsolaterally, and the spinal cord is released. Upon retraction of tangled rootlets, spinal cord traction may ensue and lead to spinal cord damage associated with a change in SSEP. Generally, MEP signal is not checked during such decompression, because of concerns over further damaging the spinal cord. In this case, a change in the SSEP signal was detected during surgery, and we were able to act appropriately to prevent damage to the spinal cord. Furthermore, an increase in the MEP signal was observed, indicating that the spinal cord had been decompressed and the motor functions were spared.
When using the posterior approach to operate on cervical spinal lesions, preservation of the C2 insertion muscle (the semispinaliscervicis muscle) reduces subsequent posterior neck pain and instability18,19). Because the main symptom experienced by our patient was posterior neck pain, it was essential that the surgery did not cause any further posterior neck pain. Therefore, suboccipital craniectomy with C1 laminectomy was performed. Since C2 laminectomy was not performed, the muscles inserting into C2 were preserved; nevertheless, the operating field was wide enough. Six months after surgery, the patient had no headache or instability.
References
1. Aryanpur J, Hurko O, Francomano C, Wang H, Carson B. Craniocervical decompression for cervicomedullary compression in pediatric patients with achondroplasia. J Neurosurg. 1990; 73:375–382. PMID: 2384775.

2. Ball BG, Krueger BR, Piepgras DG. Anomalous vertebral artery compression of the spinal cord at the cervicomedullary junction. Surg Neurol Int. 2011; 2:103. PMID: 21886876.

3. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery : a review focus on the corticospinal tracts. Clin Neurophysiol. 2008; 119:248–264. PMID: 18053764.

4. Detwiler PW, Porter RW, Harrington TR, Sonntag VK, Spetzler RF. Vascular decompression of a vertebral artery loop producing cervical radiculopathy. Case report. J Neurosurg. 1998; 89:485–488. PMID: 9724128.

5. Furumoto T, Nagase J, Takahashi K, Itabashi T, Iai H, Ishige N. Cervical myelopathy caused by the anomalous vertebral artery. A case report. Spine (Phila Pa 1976). 1996; 21:2280–2283. PMID: 8902976.
6. Grabb PA, Mapstone TB, Oakes WJ. Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery. 1999; 44:520–527. discussion 527-528. PMID: 10069589.

7. Jamjoom AB, Rawlinson JN, Coakham HB. Multiple neurological lesions due to vertebrobasilar dolichoectasia. Br J Neurosurg. 1990; 4:147–154. PMID: 2357284.

8. Kim K, Mizunari T, Kobayashi S, Ishii S, Teramoto A. [Occipital neuralgia caused by the compression of the fenestrated vertebral artery: a case report]. No Shinkei Geka. 1999; 27:645–650. PMID: 10440039.
9. Kitagawa M, Nakagawa Y, Kitaoka K, Kobayashi N, Ishikawa T, Nagashima M. [Accessory nerve paralysis due to compression of the fenestrated vertebral artery]. No Shinkei Geka. 1988; 16:1173–1177. PMID: 3205359.
10. Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007; 37:407–414. PMID: 18083496.

11. Koyama S, Maeda T, Komine A. [Medulla and upper cervical cord compression by bilateral vertebral artery presented with myelopathy and drop attack: case report]. No To Shinkei. 2002; 54:435–439. PMID: 12058415.
12. Maiuri F, Iaconetta G, Gallicchio B, Briganti F. Coiling of the vertebral artery presenting with neuralgic pain. Clin Neurol Neurosurg. 1997; 99:56–59. PMID: 9107470.

13. Morikawa K, Ohkawa N, Yamashita S. [Surgical decompression for the C-1 and C-2 sensory roots and upper cervical cord in a case with cervical myelopathy and occipital neuralgia due to bilateral fenestration of vertebral artery: a case report]. No Shinkei Geka. 1993; 21:1035–1038. PMID: 8255379.
14. Nash CL Jr, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Relat Res. 1977; (126):100–105. PMID: 598095.

15. Nishiura T, Fujiwara K, Handa A, Gotoh M, Tsuno K, Ishimitsu H. [Cervical myelopathy caused by bilateral vertebral artery compression]. No Shinkei Geka. 1998; 26:45–50. PMID: 9488991.
16. Paksoy Y, Levendoglu FD, Ogün CO, Ustün ME, Ogün TC. Vertebral artery loop formation a frequent cause of cervicobrachial pain. Spine (Phila Pa 1976). 2003; 28:1183–1188. PMID: 12782990.

17. Pico F, Labreuche J, Touboul PJ, Amarenco P. GENIC Investigators. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003; 61:1736–1742. PMID: 14694039.

18. Riew KD, Raich AL, Dettori JR, Heller JG. Neck Pain Following Cervical Laminoplasty : Does Preservation of the C2 Muscle Attachments and/or C7 Matter? Evid Based Spine Care J. 2013; 4:42–53. PMID: 24436698.
19. Sakaura H, Hosono N, Mukai Y, Fujimori T, Iwasaki M, Yoshikawa H. Preservation of muscles attached to the C2 and C7 spinous processes rather than subaxial deep extensors reduces adverse effects after cervical laminoplasty. Spine (Phila Pa 1976). 2010; 35:E782–E786. PMID: 20581755.

20. Satoh S, Yamamoto N, Kitagawa Y, Umemori T, Sasaki T, Iida T. Cervical cord compression by the anomalous vertebral artery presenting with neuralgic pain. Case report. J Neurosurg. 1993; 79:283–285. PMID: 8331414.

21. Shah A, Mahore A, Goel A. Bilateral vasculopexy of anomalous vertebral arteries causing cervicomedullary compression : case report and technical note. Eur Spine J. 2012; 21(Suppl):S505–S508. PMID: 22237850.
22. Smoker WR, Price MJ, Keyes WD, Corbett JJ, Gentry LR. High-resolution computed tomography of the basilar artery : 1. Normal size and position. AJNR Am J Neuroradiol. 1986; 7:55–60. PMID: 3082144.
23. Takahashi Y, Sugita S, Uchikado H, Miyagi T, Tokutomi T, Shigemori M. Cervical myelopathy due to compression by bilateral vertebral arteries--case report. Neurol Med Chir (Tokyo). 2001; 41:322–324. PMID: 11458746.

24. Takei H, Sagae M, Chiba K, Ogino T. The long-term follow-up of surgical treatment for cervical myelopathy with severe nape and upper arm pain caused by the anomalous vertebral artery : case report. Spine (Phila Pa 1976). 2008; 33:E611–E613. PMID: 18670330.
25. Ubogu EE, Chase CM, Verrees MA, Metzger AK, Zaidat OO. Cervicomedullary junction compression caused by vertebral artery dolichoectasia and requiring surgical treatment. Case report. J Neurosurg. 2002; 96:140–143. PMID: 11794596.

26. Watanabe K, Hasegawa K, Takano K. Anomalous vertebral artery-induced cervical cord compression causing severe nape pain. Case report. J Neurosurg. 2001; 95(1 Suppl):146–149. PMID: 11453419.

27. Zornow MH, Grafe MR, Tybor C, Swenson MR. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr Clin Neurophysiol. 1990; 77:137–139. PMID: 1690114.

Fig. 1
Sagittal T2-weighted MR images of the cervical spine (A) show the anomalous vertebral arteries (arrows) located dorsal to the spinal cord. Axial T2-weighted MR image of the C1 level (B) shows the anomalous tortuous vertebral arteries (arrows) that compressed the dorsal portion of the spinal cord bilaterally at the cervicomedullary junction. Neither congenital anomalies of the posterior fossa nor any degenerative changes to the spinal column were evident.
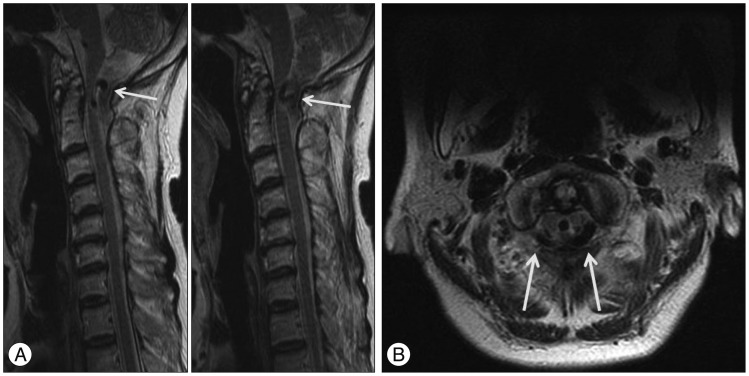
Fig. 2
CT angiography source image (A) and 3D reconstructed image (B) revealed that both vertebral arteries were elongated and enlarged. The hollow indicates the intradural segment of the elongated and enlarged vertebral arteries.
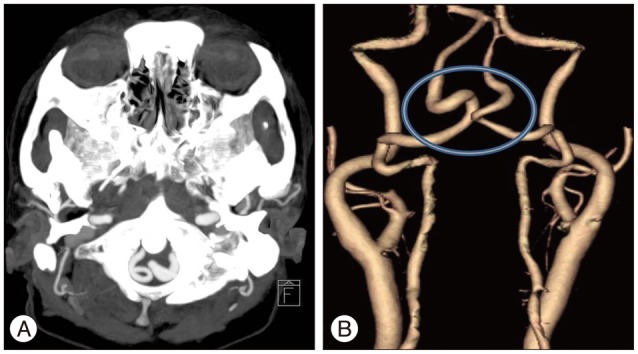
Fig. 3
Intraoperative photograph, showing the dura opened into a Y shape, and the elongated and enlarged vertebral arteries (VAs) that seemed somewhat sclerotic. Both the VAs were kissing each other. The C2 rootlets (arrows) were entangled in the VAs.
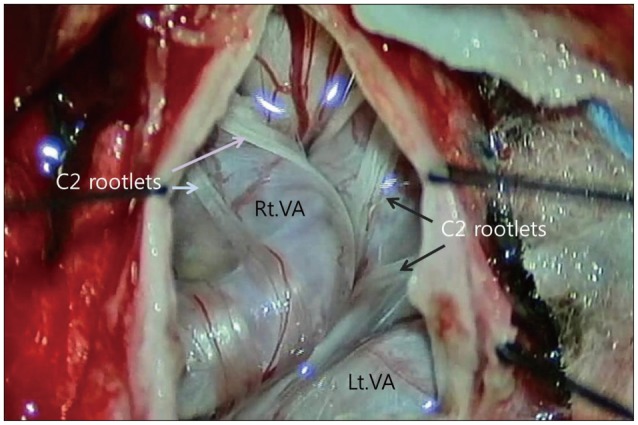
Fig. 4
Amplitude of the somatosensory evoked potential for the right tibialis anterior muscle dropped below 50% of baseline during the operation (red arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download