Abstract
Stent thrombosis is a major limitation of stent-assisted coiling, which is an effective method for treating wide-necked aneurysms. Although early in-stent thrombosis has been reported, very late stent thrombosis (VLST) (>1 year) has not been reported following implantation of a single self-expandable stent designed for coiling. Herein, the authors present a case of VLST that occurred 14 months after single stent implantation in a large paraclinoid aneurysm with an ultra-wide neck involving the parent artery circumferentially. This case indicates the need for establishing guidelines regarding the optimal duration of prophylactic antiplatelet therapy following stent-assisted coiling, which remains undefined in the neuroendovascular field.
Stent-assisted coiling is a safe and effective method for treating wide-necked aneurysms1), but ischemic stroke related to stent thrombosis is a major potential drawback. All thromboses occur within one year after the initial procedure, very late stent thrombosis (VLST), which is defined as stent thrombosis occurring one year after implantation2), following sole stent-assisted coiling has not been previously reported. Here, we report the first case of VLST, which occurred 14 months after a typical sole stent-assisted coiling procedure.
This case highlights the possible mechanism of VLST after single stent-assisted coiling and raises concerns about the duration of prophylactic antiplatelet therapy (APT).
A 56-year-old woman presented with subarachnoid hemorrhage from a ruptured aneurysm in the anterior communicating artery (AcoA) and an unruptured large aneurysm with an ultrawide neck involving the parent artery circumferentially at the right paraclinoid internal carotid artery (ICA) (Fig. 1). The ruptured AcoA aneurysm was treated successfully by clipping. Endovascular coil embolization was performed for the paraclinoid aneurysm 4 weeks after clipping. Coiling was performed using a stent-assisted technique for wide neck remodeling. The patient received oral aspirin (300 mg/d) and clopidogrel (75 mg/d) for 7 days before the coiling procedure, which was performed using a transfemoral approach under full anticoagulation with intravenous heparin (activated clotting time >250 s). First, a closed-cell Enterprise stent (4.5×37 mm; Cordis, Miami Lakes, FL, USA) was navigated in the ICA and implanted across the wide neck of the aneurysm, and coiling then was performed using 19 bare platinum coils (Orbit; Cordis, Miami Lakes, FL, USA) via a SL-10 microcatheter (Boston Scientific, Natick, MA, USA), which was jailed outside the stent. The final angiogram showed near complete obliteration of the aneurysm with minimal neck remnant (Fig. 2A).
The patient had an uneventful postoperative course and was neurologically normal at the out-patient department follow-up. Furthermore, follow-up angiography performed at 12 months after the coiling procedure depicted no change in the small amount of residual filling of the aneurysmal neck and a well-preserved parent artery without any thrombosis or stenosis (Fig. 2B).
The patient was maintained on dual antiplatelet therapy (APT) for 12 months after the initial treatment. We used 300 mg aspirin and 75 mg clopidogrel as the dual antiplatelet regimen for 4 weeks followed by 200 mg aspirin and 75 mg clopidogrel for 5 months and 100 mg aspirin and 75 mg clopidogrel for the remaining 6 months. After the 1-year follow-up angiographic evaluation, dual APT was switched to aspirin monotherapy.
Two months after switching to aspirin monotherapy, the patient experienced transient ischemic attack symptoms. Computed tomography angiography revealed occlusion of the right ICA at the level of stent implantation. Diffusion weighted imaging taken for evaluating ischemic symptoms demonstrated multiple embolic infarctions in the right hemisphere (Fig. 3A). Digital subtraction angiography (DSA) revealed in-stent thrombosis extending from the cavernous ICA to the distal ICA (Fig. 3B). The patient admitted stopping her aspirin medication prior to a dental procedure conducted 3 days before the ischemic event.
The patient was managed conservatively, because the potential risk of thrombus distal migration was considered to be high for interventional therapy. Clopidogrel 75 mg was added to aspirin 200 mg and low molecular heparin was administrated for 10 days. A follow-up angiography showed completely resolution of the thrombosis with good flow through the parent artery (Fig. 3C). The patient was discharged neurologically intact on 200 mg aspirin and 75 mg clopidogrel.
Stent-assisted coiling is increasingly being used to treat aneurysms6,11), but, although this is an effective and relatively safe technique1), it has inherent problems such as thromboembolic complications and in-stent stenosis10,18). The timing of stent thrombosis can be classified as acute (<24 h post procedure), early or subacute (24 h to 30 days post procedure), late (31 days to 1 year post procedure), or very late (>1 year post procedure)2). In-stent thrombosis within 1 year after stenting has been reported to occur at a rate of around 3% after stent-assisted coiling10,14,15,19). However, no report has previously described VLST following implantation of a sole stent, which is self-expanding intracranial stent designed for coiling assistance. The mechanisms of VLST previously proposed are; progressive in-stent stenosis, premature discontinuation of dual APT, delayed atypical response to adjacent polyglycolic acid-loaded "bioactive" coils, spontaneous occlusion of the parent artery, stenting across major arterial side branches, stent mal-apposition, stent fracture, stent strut penetration of the necrotic core, disruption of vulnerable plaque near the stent, radiation therapy, and hypersensitivity vasculitis4,7). In our case, no in-stent stenosis or stenosis around the stented vessel was noted by 1-year follow-up angiography. Furthermore, we used only bare-metal coils. Thus, premature discontinuation of antiplatelet medication was the likely cause of VLST in our case. The prevention of thromboembolic complications after stenting is currently achieved with prophylactic dual APT12), but the regimens and durations of prophylactic APT are controversial9,21). It is recognized intracranial stents are thrombogenic until stent endothelialization occurs, and that the duration of APT should be based on the completion of endothelialization. It has been reported by some that endothelialization is complete within 6 months after BMS implantation5,8,16), but no human data is available regarding the rate of stent endothelialization after stent-assisted coiling. In the interventional cardiology field, current guidelines recommend dual APT for at least 1 month for BMSs, but up to 1 year for drug-eluting stents, which cause delayed endothelialization22). However, in the neuroendovascular field, the optimal duration of prophylactic APT following stent-assisted coiling remains undefined, and clinical practices are highly discrepant; some authors have recommended only 3 or 6 months of antiplatelet therapy after stent placement for coiling17,20). These recommendations seem to be based on the facts that the target vessel for stent placement is usually nonatherosclerotic and that stents being used for coiling assistance are of bare-metal and have self-expandable properties, and thus, do not delay endothelialization over stent struts or cause endothelial injury16).
However, a much longer duration of APT may be required when stent endothelialization is only partially achieved. The occurrence of VLST may mean that formation of a complete neointimal layer incorporating stent struts has failed despite the passage of considerable time after stent implantation. Furthermore, it has been established that exposed stent struts are prone to thrombosis. Our patient had a large aneurysm with a circumferential wide neck, which suggests a large portion of the stent might have unopposed the parent vessel. In addition, it is likely that stent struts facing a circumferential wide aneurysmal neck are endothelialized at a far slower rate than that of a similar sized stents placed across a smaller aneurysmal neck. Another possible pathophysiology of incomplete endothelialization involves mechanical factors of closed-cell stents. These stents can be kinked or flattened in tortuous curves, which can result in incomplete stent apposition, causing incomplete endothelialization3,13). Thus, the ultra-wide neck involving the parent artery circumferentially and the closed-cell stent deployed in the tortuous carotid siphon probably contributed to the incomplete stent endothelialization in our case. Although we present only a single case of VLST after sole stent-assisted coiling, our observation suggest that APT is needed for much longer period after sole stent-assisted coiling for a large aneurysm with an ultra-large circumferential wide neck. A study is required to determine optimal APT regimens according to stent type, number, and aneurysm size, shape after stent-assisted coiling.
We present a case of a VLST after sole stent-assisted coiling. Although VLST is extremely rare, it is a serious event with potentially devastating consequences. This case demonstrates that long-term APT and patient strict adherence are required to possibly prevent this complication when a large portion of the stent is anticipated to be unopposed to the vessel wall.
References
1. Amenta PS, Dalyai RT, Kung D, Toporowski A, Chandela S, Hasan D, et al. Stent-assisted coiling of wide-necked aneurysms in the setting of acute subarachnoid hemorrhage : experience in 65 patients. Neurosurgery. 2012; 70:1415–1429. discussion 1429. PMID: 22186840.

2. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials : a case for standardized definitions. Circulation. 2007; 115:2344–2351. PMID: 17470709.
3. Ebrahimi N, Claus B, Lee CY, Biondi A, Benndorf G. Stent conformity in curved vascular models with simulated aneurysm necks using flat-panel CT : an in vitro study. AJNR Am J Neuroradiol. 2007; 28:823–829. PMID: 17494650.
4. Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003; 108:1701–1706. PMID: 14504181.

5. Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999; 99:44–52. PMID: 9884378.

6. Fargen KM, Hoh BL, Welch BG, Pride GL, Lanzino G, Boulos AS, et al. Long-term results of enterprise stent-assisted coiling of cerebral aneurysms. Neurosurgery. 2012; 71:239–244. discussion 244. PMID: 22472556.

7. Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK. Very late thrombosis of a pipeline embolization device construct : case report. Neurosurgery. 2010; 67(3 Suppl Operative):onsE313–onsE314. discussion onsE314. PMID: 20679914.
8. Grewe PH, Deneke T, Machraoui A, Barmeyer J, Müller KM. Acute and chronic tissue response to coronary stent implantation : pathologic findings in human specimen. J Am Coll Cardiol. 2000; 35:157–163. PMID: 10636274.

9. Grines CL, Bonow RO, Casey DE Jr, Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents : a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol. 2007; 49:734–739. PMID: 17291948.
10. Kanaan H, Jankowitz B, Aleu A, Kostov D, Lin R, Lee K, et al. In-stent thrombosis and stenosis after neck-remodeling device-assisted coil embolization of intracranial aneurysms. Neurosurgery. 2010; 67:1523–1532. discussion 1532-1533. PMID: 21107183.

11. Kim DJ, Suh SH, Lee JW, Kim BM, Lee JW, Huh SK, et al. Influences of stents on the outcome of coil embolized intracranial aneurysms : comparison between a stent-remodeled and non-remodeled treatment. Acta Neurochir (Wien). 2010; 152:423–429. PMID: 19806305.

12. King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention : a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008; 51:172–209. PMID: 18191745.

13. Krischek O, Miloslavski E, Fischer S, Shrivastava S, Henkes H. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg. 2011; 54:21–28. PMID: 21506064.

14. Lee CY, Ryu CW, Koh JS, Kim EJ. Total occlusion of the internal carotid artery by subacute in-stent thrombosis and subsequent spontaneous recanalization after stent-assisted coil embolization. Neurointervention. 2011; 6:38–41. PMID: 22125748.

15. Lin RJ, Tang SC, Jeng JS. Stroke due to late in-stent thrombosis following carotid stenting. Acta Neurol Taiwan. 2011; 20:161–162. PMID: 21739397.
16. Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005; 56:E416. discussion E416. PMID: 15670395.

17. Luo CB, Teng MM, Chang FC, Lin CJ, Guo WY, Chang CY. Stent-assisted coil embolization of intracranial aneurysms : a single center experience. J Chin Med Assoc. 2012; 75:322–328. PMID: 22824046.

18. Mocco J, Fargen KM, Albuquerque FC, Bendok BR, Boulos AS, Carpenter JS, et al. Delayed thrombosis or stenosis following enterprise-assisted stent-coiling : is it safe? Midterm results of the interstate collaboration of enterprise stent coiling. Neurosurgery. 2011; 69:908–913. discussion 913-914. PMID: 21670718.
19. Szikora I, Berentei Z, Kulcsar Z, Barath K, Berez A, Bose A, et al. Endovascular treatment of intracranial aneurysms with parent vessel reconstruction using balloon and self expandable stents. Acta Neurochir (Wien). 2006; 148:711–723. discussion 723. PMID: 16708169.

20. Thorell WE, Chow MM, Woo HH, Masaryk TJ, Rasmussen PA. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005; 56:1035–1040. discussion 1035-1040. PMID: 15854251.
21. Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting : a randomized multicenter trial. Circulation. 2012; 125:2015–2026. PMID: 22438530.

22. Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction : a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2011; 57:e215–e367. PMID: 21545940.
Fig. 1
Three-dimensional angiography showing an aneurysm at the anterior communicating artery and a large aneurysm with a wide circumferential neck (arrow) at the paraclinoid internal carotid artery.
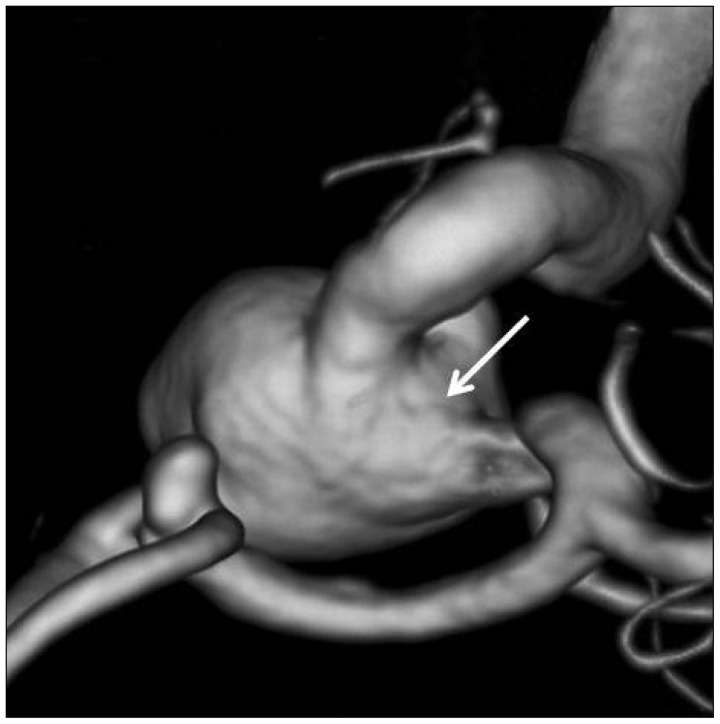
Fig. 2
A : Angiography taken immediately after coiling showing a minor amount of residual filling in the aneurysm neck and a preserved parent artery without any thrombosis. B : Same angle at 1-year follow-up angiography demonstrating no change in the small amount of residual filling of the aneurismal neck and no stenosis or thrombosis.
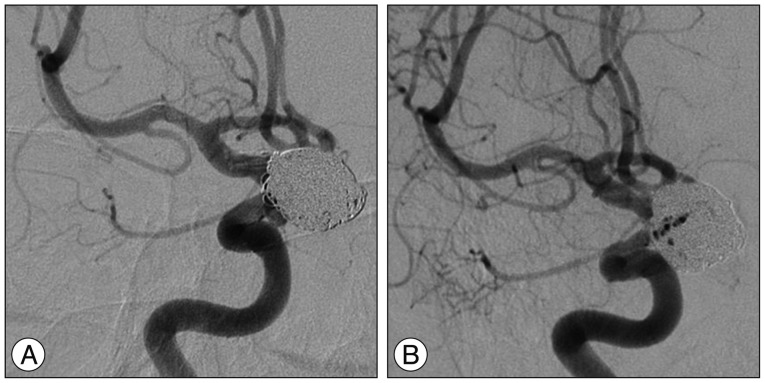
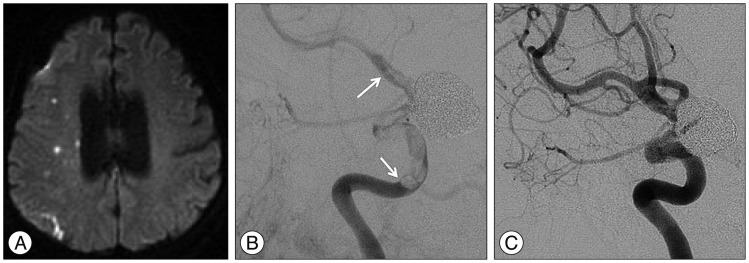
Fig. 3
A : Diffusion weighted image taken for evaluating the patient's ischemic symptoms demonstrating multiple embolic infarctions in the right hemisphere. B : Angiography obtained to evaluate the patient's ischemic symptoms showing in-stent thrombosis (arrows) extending from the cavernous internal carotid artery to the distal internal carotid artery. C : Follow-up angiography showing a completely resolved thrombosis after management with antiplatelet agents and systemic heparinization.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download