Abstract
A previously healthy 52-year-old man presented to the emergency room with acute onset left hemiparesis and dysarthria. Brain computed tomography and magnetic resonance examinations revealed acute cerebral infarction in the right middle cerebral artery territory and a sphenoid ridge meningioma encasing the right carotid artery terminus. Cerebral angiography demonstrated complete occlusion of the right proximal M1 portion. A computed tomography perfusion study showed a wide area of perfusion-diffusion mismatch. Over the ensuing 48 hours, left sided weakness deteriorated despite medical treatment. Emergency extracranial-intracranial bypass was performed using a double-barrel technique, leaving the tumor as it was, and subsequently his neurological function was improved dramatically. We present a rare case of sphenoid ridge meningioma causing acute cerebral infarction as a result of middle cerebral artery compression.
Meningiomas are prevalent brain tumors and are commonly located at the skull base2,11,13). By virtue of position, these tumors have the potential to affect portions of the internal carotid artery (ICA) and compromise cerebral blood blow. Transient ischemic attack is a known complication of skull base meningioma. However, acute cerebral infarction, resulting from intracranial arterial occlusion or obstruction related to meningioma is extremely rare4,9,13,15). Here, the authors report a case of sphenoid ridge meningioma presenting with acute cerebral infarction caused by middle cerebral artery (MCA) compression.
A 52-year-old man with no significant prior medical history was brought to our emergency room with drowsy mental status that lasted ten hours after the onset of left hemiparesis and dysarthria. The patient's left extremities were weak, and muscle power was of grade 4 in upper and lower limbs. Brain computed tomography (CT) and magnetic resonance examinations revealed acute cerebral infarction in the right MCA territory and an extra-axial mass with homogenous enhancement in the medial portion of the right sphenoid ridge (Fig. 1A, B). Magnetic resonance angiogram showed complete occlusion of the right ICA terminus (Fig. 1C). The infarction included the right uncus, insula, medial occipitotemporal gyrus, basal ganglia, corona radiate, and precentral gyrus (Fig. 1D, E). The tumor, which was consistent with a sphenoid ridge meningioma, encased and compressed the right ICA terminus. Cerebral angiography demonstrated complete occlusion of the right proximal M1 portion with slightly limited collateral circulation to the right MCA territory and a radiographic blush from the surrounding meningioma (Fig. 1F, G). Flow in the right MCA had been partially reconstituted by supply from the ipsilateral anterior cerebral artery and the posterior cerebral artery, but was much reduced. A CT perfusion study obtained shortly after arrival showed dramatic prolongation of time to peak and mean transit time of the right MCA territory, indicating obviously decreased regional cerebral blood flow in the involved territory (Fig. 1H).
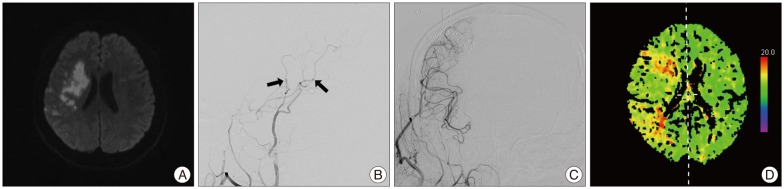
The patient was admitted and started on dual antiplatelet therapy, induced hypertension, and volume expansion. However, over the ensuing 48 hours, the left hemiparesis deteriorated steadily to muscle power grade 2 in upper and lower limbs, and follow-up magnetic resonance imaging demonstrated enlargement of the area of the diffusion weighted abnormality (Fig. 2A). Emergency extracranial-intracranial (EC-IC) bypass was performed uneventfully. We have adopted a "double-barrel" technique whereby both branches of the superficial temporal artery are joined with MCA recipients to augment flow to the whole territory of the MCA, while leaving the tumor as is (Fig. 2B, C). The patient awoke in the recovery room with exhibited a dramatic improvement of his preoperative weakness over the next 48 hours. He was maintained on oral aspirin at 100 mg/day and clopidogrel at 75 mg/day. CT perfusion scans obtained on the 14th postoperative day revealed improved cerebral blood flow in the involved territory (Fig. 2D). At the time of writing the plan was to follow the tumor. We are considering a gamma knife radiosurgery for meningioma, if needed.
The rate at which meningiomas present with symptoms of cerebral ischemia is unknown. Komotar et al.13) retrospectively reviewed the medical records of 1617 patients with meningiomas evaluated by the surgical neuropathology service at their institution from 1985 to 2001 and estimated an incidence of meningioma-related cerebral ischemia by carotid artery compression of only 0.19% (3 of the 1617 tumors).
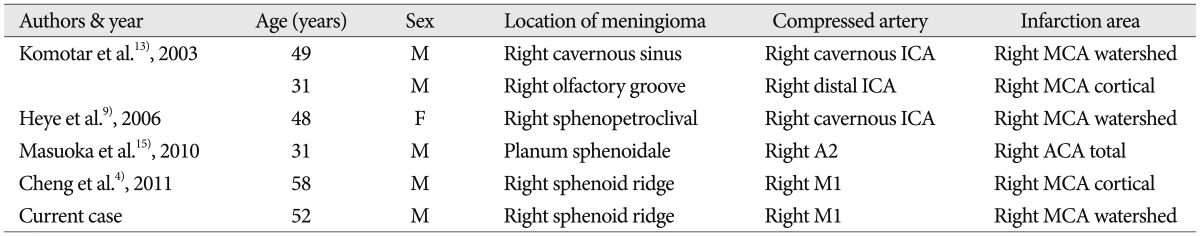
Previous reports have suggested that ICA compression by meningioma located in the skull base may produce transient neurological symptoms, such as, loss of consciousness, hemiparesis, paresthaesias, and global amnesia1,3,5,7). Nevertheless, the incidence of complete cerebral infarction resulting from arterial occlusion or obstruction related to meningioma is extremely rare4,9,13,15). We have summarized the reported cases from English literature of complete cerebral infarction caused by a meningioma in Table 1. Typically these tumors do not change vascular patency even when they completely encase the ICA and its bifurcation, because of their slow growth rates and non-invasive nature, and because of the high arterial pressure. Even when obstruction of the artery occurs due to compression, this is well compensated for by collateral flow through the circle of Willis. On the other hand, cortical veins and dural sinuses, which are low pressure compartments with thin walls, are frequently compromised by meningioma8). However, the slow growth rate of the tumor allows the development of substantial collateral drainage, and as a result, cortical infarction due to venous insufficiency has only been reported postoperatively after injury to these compensatory pathways12). In the present case, the meningioma, encased and occluded the right ICA terminus, and could have caused acute infarction of the MCA territory. Judging from the pattern of infarction observed by magnetic resonance examination, in which infarction included not only watershed zone but also cerebral cortex, the stroke was probably attributable to both hemodynamic hypoperfusion resulted from external compression by the meningioma, as well as artery to artery thromboembolism secondary to thrombus formation in stenotic artery. Furthermore, initial cerebral angiography suggested stump thrombosis at the ICA terminus. The patient had no evidence of vasculopathy or any other known stroke etiology.
Despite the discouraging results of the international randomized EC-IC bypass study in 1985 and the carotid occlusion surgery study randomized trial in 20116,17), we believe that surgical revascularization may be effective in some patients who have experienced a medication-resistant hemodynamic stroke even in the acute stage, and thus, we have continued to perform the EC-IC bypass procedure in selected patients with good results. Actually, the safety of an early EC-IC bypass in the treatment of acute ischemic stroke has not yet been fully discussed. Traditionally, surgical revascularization has been thought to be contraindicated in the presence of an acute cerebral ischemia because of reperfusion-induced hemorrhages and increased stroke rates after early revascularization. Nonetheless, there are patients who require or may benefit from early EC-IC bypass. More recent publications report promising outcomes in a subset of patients with primarily hemodynamic failure, rendering the efficacy of early EC-IC bypass contentious10,14,16). In patients with a relatively small infarction, increased perfusion/diffusion mismatch, and fluctuating or progressive symptoms resistant to medical or endovascular therapy, like the present case, early low-flow bypass may be more useful to augment blood flow in the ischemic brain while minimizing the risk of a reperfusion-related hemorrhagic complication. Furthermore, we do not hesitate to use both branches of the superficial temporal artery as donors to enhance overall hemispheric augmentation by increasing the total effective flow delivered when the ischemic penumbra area is expansive.
References
1. Araga S, Fukada M, Kagimoto H, Inagawa T, Takahashi K. Transient global amnesia and falcotentorial meningioma--a case report. Jpn J Psychiatry Neurol. 1989; 43:201–203. PMID: 2796031.
2. Bitzer M, Topka H, Morgalla M, Friese S, Wöckel L, Voigt K. Tumor-related venous obstruction and development of peritumoral brain edema in meningiomas. Neurosurgery. 1998; 42:730–737. PMID: 9574636.

3. Cameron EW. Transient ischaemic attacks due to meningioma--report of 4 cases. Clin Radiol. 1994; 49:416–418. PMID: 8045068.
4. Cheng HT, Wang CC, Chio CC, Kuo JR. Sphenoid ridge meningioma presenting as ischemia stroke. ANZ J Surg. 2011; 81:751–752. PMID: 22295325.

5. Davidovitch S, Gadoth N. Neurological deficit-simulating transient ischemic attacks due to intracranial meningioma. Report of 3 cases. Eur Neurol. 1988; 28:24–26. PMID: 3366148.

6. The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985; 313:1191–1200. PMID: 2865674.
7. Fazi S, Barthelemy M. Petroclival meningioma mimicking the presentation of a transient ischemic attack. Acta Neurol Scand. 1994; 89:75–76. PMID: 8178634.

8. Halbach VV, Higashida RT, Hieshima GB, Dowd CF, Barnwell SL. Venography and venous pressure monitoring in dural sinus meningiomas. AJNR Am J Neuroradiol. 1989; 10:1209–1213. PMID: 2512784.
9. Heye S, Maleux G, Van Loon J, Wilms G. Symptomatic stenosis of the cavernous portion of the internal carotid artery due to an irresectable medial sphenoid wing meningioma: treatment by endovascular stent placement. AJNR Am J Neuroradiol. 2006; 27:1532–1534. PMID: 16908574.
10. Horiuchi T, Nitta J, Ishizaka S, Kanaya K, Yanagawa T, Hongo K. Emergency EC-IC bypass for symptomatic atherosclerotic ischemic stroke. Neurosurg Rev. 2013; 36:559–564. discussion 564-565. PMID: 23821132.

11. Ishikawa M, Nishi S, Aoki T, Takase T, Wada E, Oowaki H, et al. Predictability of internal carotid artery (ICA) dissectability in cases showing ICA involvement in parasellar meningioma. J Clin Neurosci. 2001; 8(Suppl 1):22–25. PMID: 11386821.

12. Kiya K, Satoh H, Mizoue T, Kinoshita Y. Postoperative cortical venous infarction in tumours firmly adherent to the cortex. J Clin Neurosci. 2001; 8(Suppl 1):109–113. PMID: 11386838.

13. Komotar RJ, Keswani SC, Wityk RJ. Meningioma presenting as stroke: report of two cases and estimation of incidence. J Neurol Neurosurg Psychiatry. 2003; 74:136–137. PMID: 12486289.

14. Lee SB, Huh PW, Kim DS, Yoo DS, Lee TG, Cho KS. Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg. 2013; 115:1238–1244. PMID: 23266265.

15. Masuoka J, Yoshioka F, Ohgushi H, Kawashima M, Matsushima T. Meningioma manifesting as cerebral infarction. Neurol Med Chir (Tokyo). 2010; 50:585–587. PMID: 20671387.
16. Nussbaum ES, Janjua TM, Defillo A, Lowary JL, Nussbaum LA. Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. J Neurosurg. 2010; 112:666–673. PMID: 19499983.

17. Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011; 306:1983–1992. PMID: 22068990.

Fig. 1
A and B: Coronal and axial T1 weighted magnetic resonance images with gadolinium enhancement showing a meningioma encasing the internal carotid artery within the right cavernous sinus. C: Magnetic resonance angiogram showing complete occlusion of the right internal carotid artery terminus. D and E: Axial diffusion-weighted magnetic resonance imaging confirmed acute cerebral infarction in the right middle cerebral artery (MCA) territory, including the right uncus, insula, medial occipitotemporal gyrus, basal ganglia, corona radiate, and precentral gyrus. F and G: Cerebral angiograph with right internal carotid artery injection demonstrating complete occlusion of the right proximal M1 portion and radiographic blush from the surrounding meningioma with no significant collateral flow to the right MCA territory. H: Initial computed tomography perfusion scan showing significant asymmetry between the right and left hemispheres with striking hypoperfusion of the right MCA territory.
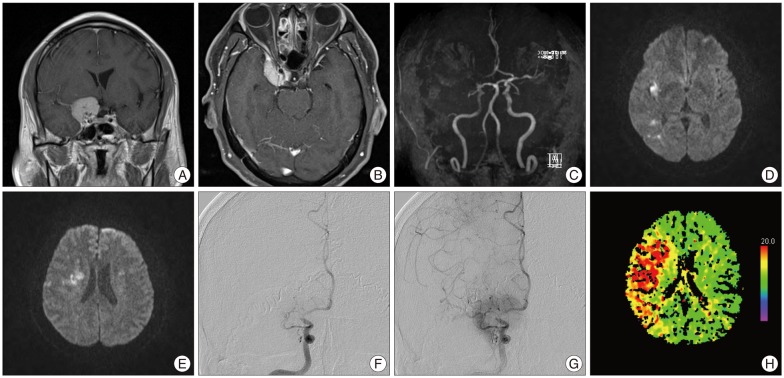
Fig. 2
A: Comparably selected axial diffusion-weighted magnetic resonance imaging obtained on Day 2 after presentation demonstrate increasing area of the diffusion weighted abnormality matching the patient's symptomatic clinical progression. Post-operative images, lateral (B) and anterior-posterior (C) view of the right external carotid angiogram showing abundant filling of multiple branches of the middle cerebral artery by patent bypasses (arrows) via the right superficial temporal artery. D: A computed tomographic perfusion scan obtained two weeks later showed shortened time to peak, indicating obviously improved cerebral blood flow after double-barrel bypass.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download