Abstract
Objective
To evaluate the incidence of postsurgical sensory complications in patients with scalp masses and classify the locations of them from a surgical standpoint according to anatomical considerations.
Methods
A total of 121 patients who underwent surgery for scalp mass were included in this study. The authors reviewed medical records and preoperative radiologic images. We investigated the complications related to sensory changes after procedure. Enrolled patients have been divided into three groups. Group A included patients with tumors above the superior nuchal line (SNL), Group B with tumors within the trapezius muscle area and patients who had tumors on the lateral trapezius muscle area were assigned to Group C. We compared the incidence related to postoperative sensory complications and summarized their additional treatments for these with clinical outcome.
Results
There were 12 patients (10%) with sensory complications related on the mass excision site (Group A: 1 patient, Group B: 2 patients, Group C: 9 patients). Six patients were affected with lesser occipital nerve (LON), 2 patients on greater occipital nerve (GON) and 4 patients on GON and LON. Over 6 months after surgery, two of the twelve patients with sensory complications did not have complete recovered pain in spite of proper medications and local chemical neurolysis with 1.0% lidocaine and dexamethasone.
Scalp masses are commonly encountered by clinicians. There are some reports regarding the incidence and prevalence of scalp masses that occur in infants or children13,24). However, in adults, because of their benign characteristic and good prognosis13), most of the literatures were reports of cases that tend to concentrate on atypical presentation of tumors and there has been no systemic analysis of them. Despite its corresponding rarity of data in the literature, scalp masses were usually easily treated and cured by surgical excision in most cases.
Most complications related to surgical excision are infection, wound dehiscence, pain, or discomfort from a skin incision. These complications were usually resolved in a few months and did not remain in any prolonged problem. However, some patients occasionally suffer from transient sensory changes including numbness, paresthesia, and dysesthesia along the sensory distributions after excision of the mass. In this study, we present our experience about sensory complications after excision of scalp masses, and evaluated predicting factors associated with sensory complications.
Between January 2009 and December 2012, we performed 121 cases of surgical removal of scalp masses. All patients had preoperative CT scan routinely to evaluate its characteristics, including the location and pathological confirmation of the tumors. A retrospective medical record review and preoperative radiologic images were analyzed.
In particular, we investigated sensory complications such as any sensory changes after procedure and defined occipital neuralgia (ON) as followed by international headache society: intermittent stabbing pain, with or without persistent aching, in the distribution of the greater, lesser or third occipital nerves; tenderness over the affected nerve; temporary pain relief of 50% or more was considered a positive response to local anesthesia injection of the affected nerve12). Enrolled patients have been divided into three groups, according to the anatomical locations of their tumor. Group A included patients with tumors above the superior nuchal line (SNL), Group B with tumors within the trapezius muscle area and patients who had tumors on the lateral trapezius muscle area were assigned to Group C (Fig. 1). If the tumor was located in borderline between the two groups, we classified into the group according to the degree of involvement in the area based on the surface of the tumor from a CT scan. We compared the risk of complication related to postoperative sensory changes with each group and summarized additional treatments for these complications with clinical outcome. Pain was assessed using a Visual Analogue Scale (VAS) was used after surgery and at follow-up for outcome measurement. Complete relief was defined as the patient having VAS 3 less. All investigations were performed in accordance with our institutional guidelines that comply with all international laws and policies.
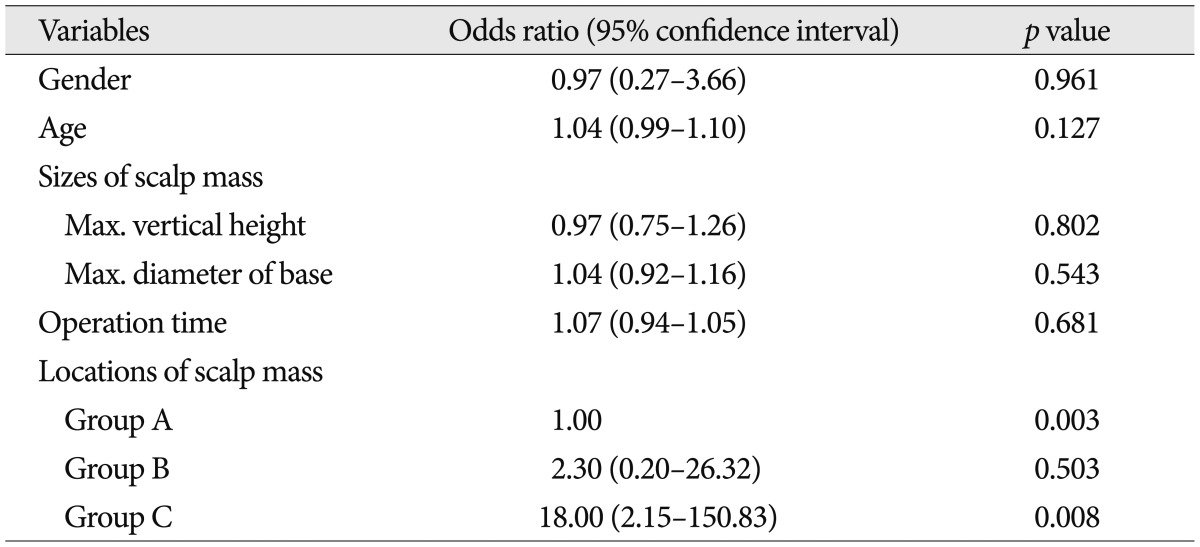
Preoperative data were evaluated using a one-way ANOVA test in case of metric data and a Pearson's chi-square test in case of nominal data to ensure that three groups of patients were comparable before surgical excision. The following variable data were analyzed by multiple logistic regression analysis with factors associated with neuropathy as the dependent variable: age, gender, tumor sizes, operation times, and groups. Null hypotheses of no difference were rejected if p-values were less than 0.05. Data were analyzed using the SPSS 12.0 statistical software (SPSS V12.0K, SPSS Inc., Chicago, IL, USA).
The median age at diagnosis was 44 years (range, 5-76 years). There were 67 males (55%) and 54 females (45%). We classified patients with three groups; Group A (n=47), Group B (n=42), and Group C (n=32). A summary of the clinical characteristics and demographic data of the group populations is in Table 1. The three groups did not differ significantly in age, gender distribution, maximal sizes of masses and operation time.
There were 12 patients (10%) with sensory complications related on the mass excision site (Group A: 1 patient, Group B: 2 patients, Group C: 9 patients). Six patients were affected with LON, 2 patients on GON and 4 patients on GON and LON. The presentations of neuropathy were numbness on 4 patients, dysesthesia on 3 patients, paresthesia on 2 patients and occipital neuralgia on 3 patients (lipoma; 2 patients, dermoid cyst; 1 patient). The follow-up period for these patients ranged from 1 to 11 months with an average of 3.5 months. Ten patients with neuropathy clearly recovered within three months. Over 3 months from surgery, two of three patients with occipital neuralgia did not have complete pain relief in spite of proper medications and local chemical neurolysis with 1.0% lidocaine and dexamethasone.
In this study, evaluation of variables was carried out using a multiple logistic regression analysis as shown in Table 3. Age, gender, size of mass, and operation time were not correlated with occipital neuropathy after mass excision. The only location of the tumor remained independently associated with probability of this presentation. The location of group C had an odds ratio of 18.00, meaning that a patient having one scalp mass in this area would be 18.00 times as likely to get complication related neuropathy as another patient not in this area (p<0.01).
Neuropathy is damage to the nerve of the peripheral nervous system, which may be caused by diseases or trauma to the nerve. Pain associated with neuropathy is described in many presentations, such as the following; burning, electric-like, increased sensitivity to touch stimulation. ON is a subtype of headache that involves the greater, lesser, and third occipital nerve distribution. Their clinical symptoms include sharpen, burning, and throbbing pain that are often unilateral and constant with paroxysmal pain1).
GON is the medial branch of the posterior primary ramus of the cervical spinal nerve and arises from between the first and second cervical vertebrae, along with LON. It proceeds between the inferior oblique and semispinalis capitis muscle, and then penetrates through the trapezius muscle to join the occipital artery. Along its course, this nerve ascends to innervate the skin of the posterior scalp. Under the trapezius muscle, the medio-posteior branch of the third cervical nerve emits a branch called TON, which penetrates the trapezius and ends in the skin of the posterior lower scalp. LON is composed of branches off of the posterior divisions of the second and third cervical nerves. Its course is described as running lateral to GON, crossing over the sternocleidomastoid muscle (SCM), and proceeding superolaterally toward the region behind and above the auricle2,17,23).
In most cases, ON originates from occipital nerves irritation by the paraspinal tissue, but recurrent ON with headache may have underlying cervical degenerations, such as cervical disc disease, and rheumatoid arthritis1,10,11). Vascular abnormalities are rare, but described pathologies include serious problems such as carotid and vertebral artery dissections3,16). Mechanical causes, such as atlanto-axial instability, subaxial instability, and atlanto-axial fusion surgery, have been reported to potentially result in concomitant ON5,9,11). Among the occipital nerves, GON is more frequently affected (90%) than LON (10%), and TON is rarely affected4).
ON related to the excision of scalp mass was not reported in the literature and it can occur by direct nerve injury during surgery or indirect nerve entrapments, such as postoperative scar tissue or adhesion8). Postoperative adhesions have been defined as abnormal fibrous connections, which join tissue surfaces in abnormal locations. In case of general surgery, abdominopelvic adhesion is a major cause of small bowel obstruction and infertility in female. Diamond et al.6) reported conditions of chronic pain, organ dysfunction, and difficult re-operations representing a general pathological process that may affect almost all parts of the human anatomy. About cellular mechanism of postoperative adhesion development, Shavell et al.20) analyzed hypoxia appears to be the crucial factor in the development of adhesion. Hypoxia triggers a cascade of intracellular mechanisms initiating the manifestation of the adhesion phenotype. These mechanisms include the increasing of anaerobic glycolysis and glucose uptake, the activation of collagen synthesis and angiogenesis by lactate and nitrogen oxide, the inhibition of apoptosis, and the stimulation of collagen production.
In this study, twelve patients were experiencing occipital neuropathy after surgery. Numbness was developed within 24 hours, and other presentations such as dysesthesia, paresthesia, ON occurred on the 2nd to 5th day which are corresponding with postoperative inflammatory reaction periods. Occipital neuropathies occurring in GON or LON were not severe to interfere with the daily life in 8 patients, but three patients with ON along LON distribution had difficulties with activities of daily living to require additional treatments. LON is more affected by surgery, not GON or TON. These complications occurred most in Group C.
We have assumed three considerations that is more affected in LON and Group C (Fig. 2).
The LON may be easily vulnerable because of its superficial course.
The LON runs vertically upward along the posterior margin of the SCM as a single trunk after it pierced the deep cervical fascia23). Tahir et al.21) reported the use of a horseshoe-shaped headrest during beach-chair surgery caused a permanent injury to the LON, and Park and Kim18) reported three patients with neuropraxia of LON after shoulder arthroscopy. They believed the etiology of injury to LON was an entrapment effect from the headrest compression and recommended that the auricle be protected with cotton or gauze during surgery in the beach-chair position. Though irrelevant to compression injury in this study, LON exposed superficially may also easily be affected from indirect irritations, such as postoperative scar tissue or suture material. However, on cadaveric study, GON was found to emerge below to average 30 mm from the occipital protuberance17).
Group A has less complications related neuropathy because of its lesser soft tissue and abundant sensory nerves.
Adhesions may result from prolonged restriction, surgical procedure, an injury causing soft tissue inflammation6,20,22). SNL is posterior margin of the scalp, which is composed of thin five layers. It contains abundant nerves that are densely innervated. Relatively below SNL, there are many posterior neck muscles and soft tissues. The trapezius muscle, splenius capitis muscle, SCM, and occipitalis muscle attached to this line. The benign masses on the scalp were usually present in the skin and a thin layer of fat and fibrous tissue. Though the neural structures supplying the scalp traverse through the deep subcutaneous tissues overlying, transection of these sensory nerves does not result in serious complication, and the recovery of peripheral sensory function after direct injury is typical.
Localizations of masses were rare in midline locations relation with Group A and B.
Mosser et al.17) analyzed the course of GON and reported the mean distance of emergence of GON from the midline was 15 mm on average. Park et al. studied an analysis of clinical data, including incidence, clinical manifestations and surgical treatment of neoplasm of the scalp and calvarium in adults13). They evaluated sixty seven patients with masses and among them, only seven lesions (dermoid 1; epidermoid 2; lipoma 3; osteoma 1) occurred in the midline. In this study, only five patients (4%) had midline locations (epidermoid 4; lipoma 1). If the incidence of midline location is more frequent than lateral location, GON seems to be easily affected as much as LON.
The classification system is based on the location of scalp masses have not been reported; therefore, we tried to classify three groups of locations for scalp masses in accordance with our results. Rationales for our classification were summarized as the following:
1) Patient's condition of being in ON sometimes can seriously interfere with daily life.
2) The occipital nerves are sensory nerves, which enter the spinal cord through the tract of Lissauer to terminate the substantia gelatenosa of the upper cervical cord. Anatomical variations may exist, but GON emerge through the trapezius muscle and LON proceeds along the posterior margin of SCM at all time7).
3) SCM attached to the lateral half of SNL and the trapezius muscle, medial third of SNL. We can easily examine the anatomical boundary between GON and LON.
4) Above SNL, there are abundant small branches of the occipital nerves or trigeminal nerves, not the main trunk.
Most scalp masses are cured with surgery, and during excision, there are no critical problems, with the exception of direct neural and arterial injury. However, in cases of patients with postsurgical sensory complications, if clinical manifestations were mild, these were recovered within several months. However, if ON after surgery occurs as these three patients, we should prescribe medications and perform chemical neurolysis to patients for relief of pain15). According to recent studies, we also recommend pulsed radiofrequency neuromodulation, neurectomy, and surgical neurolysis, which are considerable treatment modalities4,14), or after removal of the tumor located in Group C, the attempt to use the anti-adhesive material also seemed to help preventing adhesion related complications.
According to our results, postsurgical occipital neuropathy should be considered as a complication related to the excision of scalp mass. This complication is more frequent in Group C because of anatomical characteristics of the occipital nerves and there were no statistical difference for other variables. For this reason, if scalp mass located at Group C, we suggest that surgeon should be aware of the possibility of this potential complications and also understand the appropriate treatment and clinical prognosis. Further controlled prospective studies are necessary to evaluate the exact anatomical relationship and clinical outcomes.
Acknowledgements
This study was supported by BioGreen21 Program (PJ009051) of Rural Development Administration.
References
1. Ahn NU, Ahn UM, Ipsen B, An HS. Mechanical neck pain and cervicogenic headache. Neurosurgery. 2007; 60(1 Supp1 1):S21–S27. PMID: 17204881.
2. Arai T, Ishikawa K, Saito T, Hashimoto Y, Asai T, Okuda Y. Distance from the external occipital protuberance to the occipital artery for occipital nerve block. J Anesth. 2013; 27:801–802. PMID: 23475441.

3. Biousse V, D'Anglejan-Chatillon J, Massiou H, Bousser MG. Head pain in non-traumatic carotid artery dissection: a series of 65 patients. Cephalalgia. 1994; 14:33–36. PMID: 8200023.

4. Choi HJ, Oh IH, Choi SK, Lim YJ. Clinical outcomes of pulsed radiofrequency neuromodulation for the treatment of occipital neuralgia. J Korean Neurosurg Soc. 2012; 51:281–285. PMID: 22792425.

5. Conroy E, Laing A, Kenneally R, Poynton AR. C1 lateral mass screw-induced occipital neuralgia: a report of two cases. Eur Spine J. 2010; 19:474–476. PMID: 19856190.

6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001; 7:567–576. PMID: 11727865.

7. Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009; 123:859–863. discussion 864. PMID: 19319048.

8. Gille O, Lavignolle B, Vital JM. Surgical treatment of greater occipital neuralgia by neurolysis of the greater occipital nerve and sectioning of the inferior oblique muscle. Spine (Phila Pa 1976). 2004; 29:828–832. PMID: 15087807.

9. Gunnarsson T, Massicotte EM, Govender PV, Raja Rampersaud Y, Fehlings MG. The use of C1 lateral mass screws in complex cervical spine surgery: indications, techniques, and outcome in a prospective consecutive series of 25 cases. J Spinal Disord Tech. 2007; 20:308–316. PMID: 17538356.

10. Haldeman S, Dagenais S. Cervicogenic headaches: a critical review. Spine J. 2001; 1:31–46. PMID: 14588366.
11. Hammond SR, Danta G. Occipital neuralgia. Clin Exp Neurol. 1978; 15:258–270. PMID: 756019.
12. International Headache Society Headache Classification Subcommittee. The International Classification of Headache Disorders. ed 2. Oxford: Blackwell Pub;2004.
13. Jeon SJ, Park SH, Ryu KS, Cho BM, Oh SM. Clinical and radiological analysis of scalp masses. J Korean Neurosurg Soc. 2002; 32:559–563.
14. Jung SJ, Moon SK, Kim TY, Eom KS. A case of occipital neuralgia in the greater and lesser occipital nerves treated with neurectomy by using transcranial Doppler sonography: technical aspects. Korean J Pain. 2011; 24:48–52. PMID: 21390179.

15. Jürgens TP, Müller P, Seedorf H, Regelsberger J, May A. Occipital nerve block is effective in craniofacial neuralgias but not in idiopathic persistent facial pain. J Headache Pain. 2012; 13:199–213. PMID: 22383125.

16. Lucchesi C, Puglioli M, Gori S. Occipital neuralgia: a symptomatic case caused by an abnormal left vertebral artery. Neurol Sci. 2013; 34:243–245. PMID: 22311642.

17. Mosser SW, Guyuron B, Janis JE, Rohrich RJ. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004; 113:693–697. discussion 698-700. PMID: 14758238.

18. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005; 21:631. PMID: 15891736.

19. Rhee WT, You SH, Kim SK, Lee SY. Troublesome occipital neuralgia developed by c1-c2 harms construct. J Korean Neurosurg Soc. 2008; 43:111–113. PMID: 19096615.

20. Shavell VI, Saed GM, Diamond MP. Review: cellular metabolism: contribution to postoperative adhesion development. Reprod Sci. 2009; 16:627–634. PMID: 19293132.

21. Tahir M, Corbett S. Lesser occipital nerve neurotmesis following shoulder arthroscopy. J Shoulder Elbow Surg. 2013; 22:e4–e6. PMID: 23352479.

22. Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain MZ, Rosen N, et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003; 11:504–509. PMID: 14617293.

23. Tubbs RS, Salter EG, Wellons JC, Blount JP, Oakes WJ. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007; 20:235–238. PMID: 16944523.

24. Yoon SH, Park SH. A study of 77 cases of surgically excised scalp and skull masses in pediatric patients. Childs Nerv Syst. 2008; 24:459–465. PMID: 17987301.

Fig. 1
To divide three groups according to anatomical locations of the scalp mass, there are two landmarks; superior nuchal line (SNL) and lateral margin of trapezius muscle (TPZ) indicated by black arrow. Group A: scalp mass located above the SNL. Group B: scalp mass existed within lateral margins of TPZ and below SNL. Group C: scalp mass located on neighbored TPZ and below SNL.
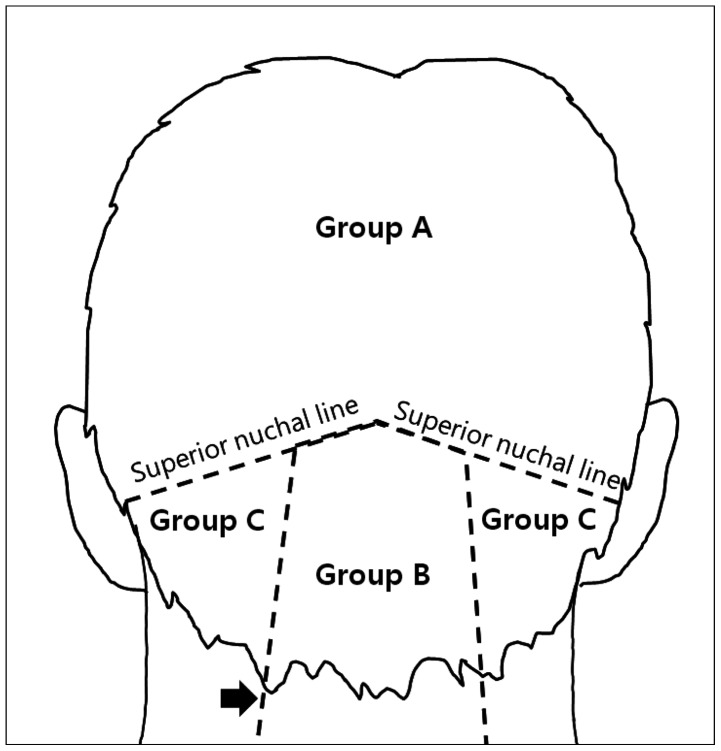
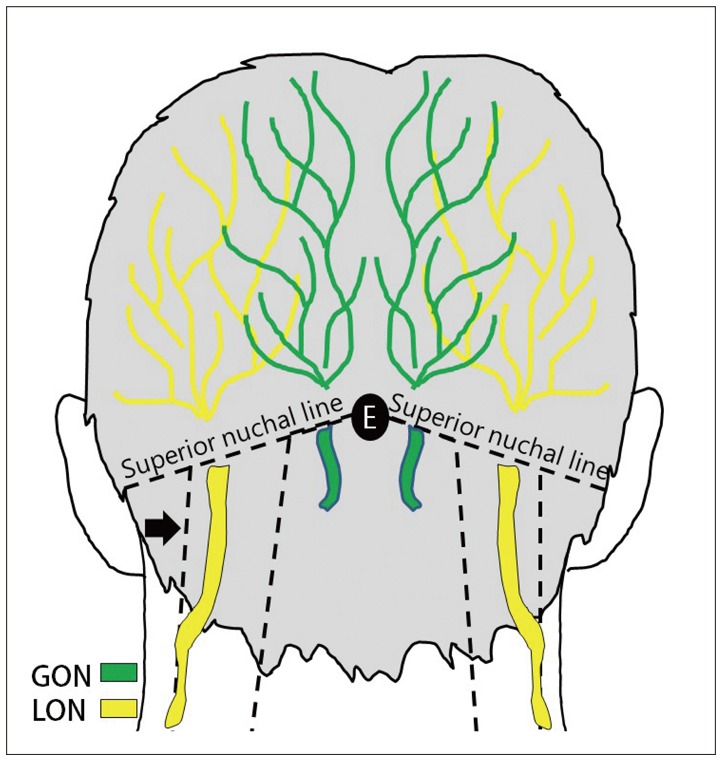
Fig. 2
We have explained anatomical considerations more affecting in the group C using schematic illustration of the occipital nerves. The main trunk of LON proceeds superficially along posterior margin of the sternocleidomastoid muscle (black arrow). The GON was found to emerge below to 30 mm from the occipital protuberance and laterally 15 mm from the midline on average. There are abundant sensory nerves above the superior nuchal line. GON: greater occipital nerve, LON: lesser occipital nerve, E: external occipital protuberance.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download