Abstract
Ganglioglioma is an infrequent tumor of the central nervous system (CNS); mostly supratentorial region. But, they can occur anywhere in the central nervous system such as brainstem, cerebellopontine angle (CPA), thalamus, optic nerve and spinal cord. Although it occurs rarely, ganglioglioma should be included in the differential diagnosis of a posterior fossa mass because early recognition is important for treatment and patient counseling.
Ganglioglioma is usually a benign slowly-growing neoplasm, and it mainly affects older children and young adults. It accounts for approximately 0.4% of all CNS tumors. It occurs most commonly in the supratentorial region; mostly in the temporal lobe (up to 85%) presenting with long-standing intractable seizures3,9,10,11).
Nevertheless, there are occasional reports of gangliogliomas occurring in the brainstem, cerebellopontine angle (CPA), thalamus, optic nerve and spinal cord. According to a review of literature, there are only 46 cases of brainstem ganglioglioma. In addition, only few cases have been studied with a magnetic resonance imaging (MRI) scan5,13,14). For these reasons, definite pathognomic radiological features and adjuvant treatment still controversy.
We experienced a rare case of ganglioglioma occurring in brainstem, high cervical cord and CPA in a child. Here, we report our case with a review of literature.
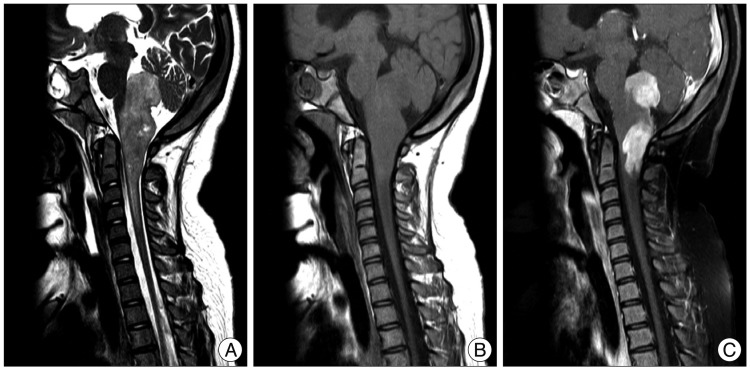
A 10-year-old girl was referred to our hospital due to repetitive aspiration pneumonia and seizure lasting over a month. On neurological examination, the patient had a slightly drowsy mental status concurrently with a bilateral lower cranial nerve palsy, horizontal nystagmus and ataxia. Computed tomography (CT) and magnetic resonance imaging (MRI) scans demonstrated a well enhanced mass on the dorsal surface of the brainstem (pons and medulla) and high cervical cord with an exophytic growth posteriorly to left CPA. This mass had a heterogeneous intensity, a slight hyperintensity on T2-weighted images and an isointensity on T1-weighted ones with a contrast enhancement (Fig. 1).
The patient received antibiotics for the treatment of pneumonia, followed by tracheostomy and -nasogastric tube feeding for the prevention of aspiration. Two weeks later, the patient underwent surgery through a midline suboccipital craniotomy and a laminectomy down to the C2 level. We performed a removal of the fourth-ventricle mass and left CPA mass near totally, but we could perform only a partial removal of the tumor attached at the posterior aspect of the medulla and high cervical cord.
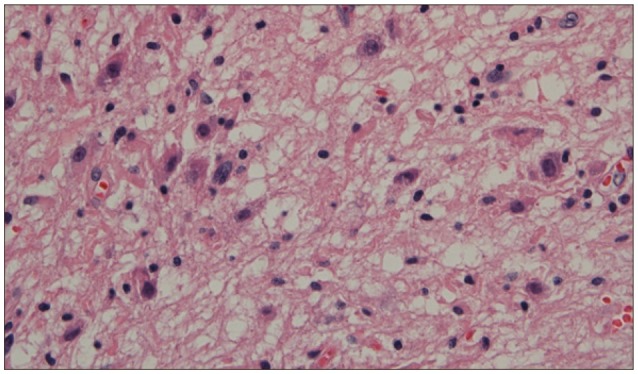
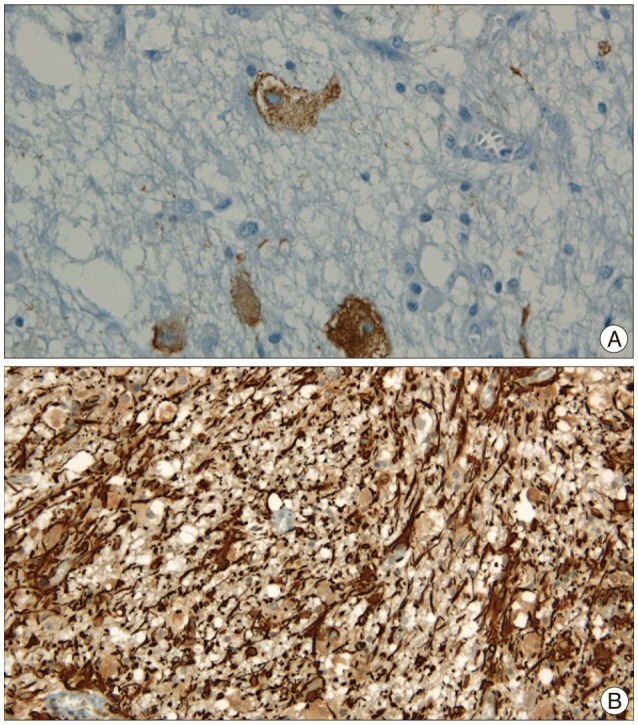
Histopathologic examinations revealed the presence of low grade ganglioglioma, which was composed of mature and immature ganglion cells without normal perineuronal oligodendrocytes (Fig. 2). On immunohistochemistry, the glial cells were positive for glial fibrillary acid protein (GFAP) and synaptophysin, chromagranin and S100 (Fig. 3).
On postoperative day 7, the patient had a neurologic deterioration due to obstructive hydrocephalus. The patient therefore underwent placement of the extraventricular drainage catheter. Two weeks later, the patient underwent ventriculo-peritoneal shunt. Postoperatively, the patient presented with a recovery of consciousness but developed the temporary right hemiparesis (Grade IV+) and sleep apnea, both of which were gradually resolved.
At a 10-month follow-up, the patient had a persistent presence of neurologic deficits such as a lower cranial palsy (lack of gag reflex) and a mild gait imbalance.
Ganglioglioma is account for 0.4-7.6% of pediatric CNS neoplasms and up to 1.3% of those in adults4). in addition, infratentorial region such as brain stem, CPA, spinal cord is extremely rare location. But, they are thought to be low malignancy potential with a benign clinical course. Therefore, ganglioglioma should be considered in the differential diagnosis of brainstem tumor. Our case is notable in that it was located in the brainstem, high cervical cord and CPA.
The supratentorial cortical lesions are often characterized by a long history of seizures, whereas posterior fossa gangliogliomas feature focal neurological deficits, cranial nerve palsy, hydrocephalus, increased intracranial pressure, speech or gait changes and myoclonus. In addition, the interval from symptom onset to the diagnosis is significantly shorter as compared with the supratentorial ganglioglioma because the infratentoraial compartment is an anatomically narrow space2). Epileptic seizures are defined as excessive paroxysmal discharges from nerve cells, leading to clinical and electrical change, that commonly arise from the cerebral cortex. Recent studies have well documented, however, that epileptic seizures of subcortical origin also occurred in humans with hypothalamic hamartomas and infratentorial ganglioglioma accompanied by cerebellar seizure1). In our case, the patient had a history of generalized seizure but did not present with it postoperatively. Presumably, this indicates that epileptic seizures might originate from the subcortical structures.
Radiographically, there is a great variability in the imaging characteristics of ganglioglioma and it is difficult to distinguish the other brainstem tumor such as subependymomas, ependymomas, or brain stem glioma5). Nevertheless, the diagnosis of brainstem ganglioglioma is great importance because of its comparatively better prognosis than other tumors in this location. Generally speaking, it usually shows an iso- to slightly hypo- signal intensity on T1-weighted MR images and hypersignal intensity on T2-weighted images. Degree of contrast enhancement after the administration of gadolinium varies, ranging from no-enhancement to marked, heterogeneous enhancement4,9). According to literature, malignant ganglioglioma tend to demonstrate more contrast enhancement. In addition, advanced MRI techniques, such as diffusion tensor imaging, proton magnetic spectroscopy, dynamic susceptibility-weighted contrast-enhanced perfusion images are useful in characterizing neoplasm in vivo, sometimes predicting tumor grade and outcome in glial brain tumors7).
Histopathologically, ganglioglioma is characterized by an intimate mixture of abnormal neuronal and glial elements. It is difficult, however, to identify it on histopathology because its reported incidence is relatively lower. This often leads to misdiagnosis of glioma. Immunohistochemical study aids the confirmation of diagnosis of ganglion cell tumor. The glial populations are reactive for GFAP, S-100 protein, and vimentin, but the neurons are for synaptophysin and Chromogranin A5,9,12). Consequently, correct diagnosis of ganglioglioma requires appropriate sampling and elaborate histologic examinations.
The gold-standard treatment for ganglioglioma is a gross total resection that associates with good prognosis. However, total resection of brainstem gangliogliomas is usually difficult because, brainstem ganglioglioma most often invades the spinal cord or the fourth ventricle that are involved in major cardiopulmonary events. Therefore, brainstem ganglioglioma shows a less favorable outcome as compared with supratentorial one2,5,11,12,15).
Nevertheless, a good long-term prognosis can be expected despite a partial removal in cases of brainstem ganglioglioma. According to a retrospective analysis, the 5-year survival rates for cerebral hemisphere, spinal cord, and brain-stem gangliogliomas were 93%, 84%, and 73%. In addition, surgical resection was more effective than biopsy and irradiation6). Recently, Zhang et al.16) have shown favorable prognosis in 7 brainstem ganglioglioma patients. Subtotal resection was achieved in 2 patients and partial resection in 5 patients. Adjuvant therapies were not administrated. Nevertheless, all patient symptoms were resolve or stable without aggravation, and MRI showed that the size of residual lesions was unchanged after 21-69 months after surgery.
There is still a controversy as to the postoperative use of chemotherapy and radiotherapy. Ganglioglioma has a good prognosis and rarity. Also, radiation therapy would influence development of the central nervous system in children. For these reasons, the study of radiotherapy and chemotherapy to brainstem ganglioglioma is not focused. Although the benefit of radiotherapy is inconclusive, it can be given to patients with a tumor histology showing anaplastic features or oligodendroglial-like cells and recurrence.
Infratentorial ganglioglioma is a very rare entity with a rare location. Nevertheless, ganglioglioma should be included in the differential diagnosis of infratentorial tumor in children. A gross total resection of it cannot be achieved, although survival benefit can be obtained through a more radical resection.
References
1. Chae JH, Kim SK, Wang KC, Kim KJ, Hwang YS, Cho BK. Hemifacial seizure of cerebellar ganglioglioma origin : seizure control by tumor resection. Epilepsia. 2001; 42:1204–1207. PMID: 11580771.

2. Chan MH, Kleinschmidt-Demasters BK, Donson AM, Birks DK, Foreman NK, Rush SZ. Pediatric brainstem gangliogliomas show overexpression of neuropeptide prepronociceptin (PNOC) by microarray and immunohistochemistry. Pediatr Blood Cancer. 2012; 59:1173–1179. PMID: 22706982.

3. Jang EW, Cho JH, Chang JH, Ahn JY. Cerebellar ganglioglioma in an old patient. J Korean Neurosurg Soc. 2007; 42:53–55.
4. Kwon JW, Kim IO, Cheon JE, Kim WS, Chi JG, Wang KC, et al. Cerebellopontine angle ganglioglioma : MR findings. AJNR Am J Neuroradiol. 2001; 22:1377–1379. PMID: 11498430.
5. Lagares A, Gómez PA, Lobato RD, Ricoy JR, Ramos A, de la Lama A. Ganglioglioma of the brainstem : report of three cases and review of the literature. Surg Neurol. 2001; 56:315–322. discussion 322-324. PMID: 11750003.
6. Lang FF, Epstein FJ, Ransohoff J, Allen JC, Wisoff J, Abbott IR, et al. Central nervous system gangliogliomas. Part 2 : Clinical outcome. J Neurosurg. 1993; 79:867–873. PMID: 8246055.
7. Löbel U, Ellison DW, Shulkin BL, Patay Z. Infiltrative cerebellar ganglioglioma : conventional and advanced MRI, proton MR spectroscopic, and FDG PET findings in an 18-month-old child. Clin Radiol. 2011; 66:194–201. PMID: 21216337.

8. Manning HL, Leiter JC. Respiratory control and respiratory sensation in a patient with a ganglioglioma within the dorsocaudal brain stem. Am J Respir Crit Care Med. 2000; 161:2100–2106. PMID: 10852794.

9. Milligan BD, Giannini C, Link MJ. Ganglioglioma in the cerebellopontine angle in a child. Case report and review of the literature. J Neurosurg. 2007; 107(4 Suppl):292–296. PMID: 17941493.
10. Mpairamidis E, Alexiou GA, Stefanaki K, Sfakianos G, Prodromou N. Brainstem ganglioglioma. J Child Neurol. 2008; 23:1481–1483. PMID: 19073857.

11. Park SH, Kim E, Son EI. Cerebellar ganglioglioma. J Korean Neurosurg Soc. 2008; 43:165–168. PMID: 19096627.

12. Shin JJ, Oh SH, Yoon DH, Kim TS. Cervical ganglioglioma. J Korean Neurosurg Soc. 2001; 30:239–243.
13. Shin MJ, Yang KH, Kim TS, Choi JU. Ganglioglioma of the brain stem : case report. J Korean Neurosurg Soc. 2002; 32:279–282.
14. Westwood DA, MacFarlane MR. Pontomedullary ganglioglioma : a rare tumour in an unusual location. J Clin Neurosci. 2009; 16:108–110. PMID: 19013803.
15. Wetmore C. Location, location, location-gene expression studies of brainstem ganglioglioma : a rare tumor in a very rare location. Pediatr Blood Cancer. 2012; 59:1153–1154. PMID: 22976749.

16. Zhang S, Wang X, Liu X, Ju Y, Hui X. Brainstem gangliogliomas : a retrospective series. J Neurosurg. 2013; 118:884–888. PMID: 23373800.
Fig. 1
MRI findings. A : T2-weighted images showing slightly high signal intensity mass on the dorsal surface of brainstem and high cervical cord. B and C : T1-weighted images showing an isointensity with a well-enhanced mass.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download