Abstract
Objective
Although surgical techniques for clipping paraclinoid aneurysms have evolved significantly in recent times, direct microsurgical clipping of large and giant paraclinoid aneurysms remains a formidable surgical challenge. We review here our surgical experiences in direct surgical clipping of large and giant paraclinoid aneurysms, especially in dealing with anterior clinoidectomy, distal dural ring resection, optic canal unroofing, clipping techniques, and surgical complications.
Methods
Between September 2001 and February 2012, we directly obliterated ten large and giant paraclinoid aneurysms. In all cases, tailored orbito-zygomatic craniotomies with extradural and/or intradural clinoidectomy were performed. The efficacy of surgical clipping was evaluated with postoperative digital subtraction angiography and computed tomographic angiography.
Results
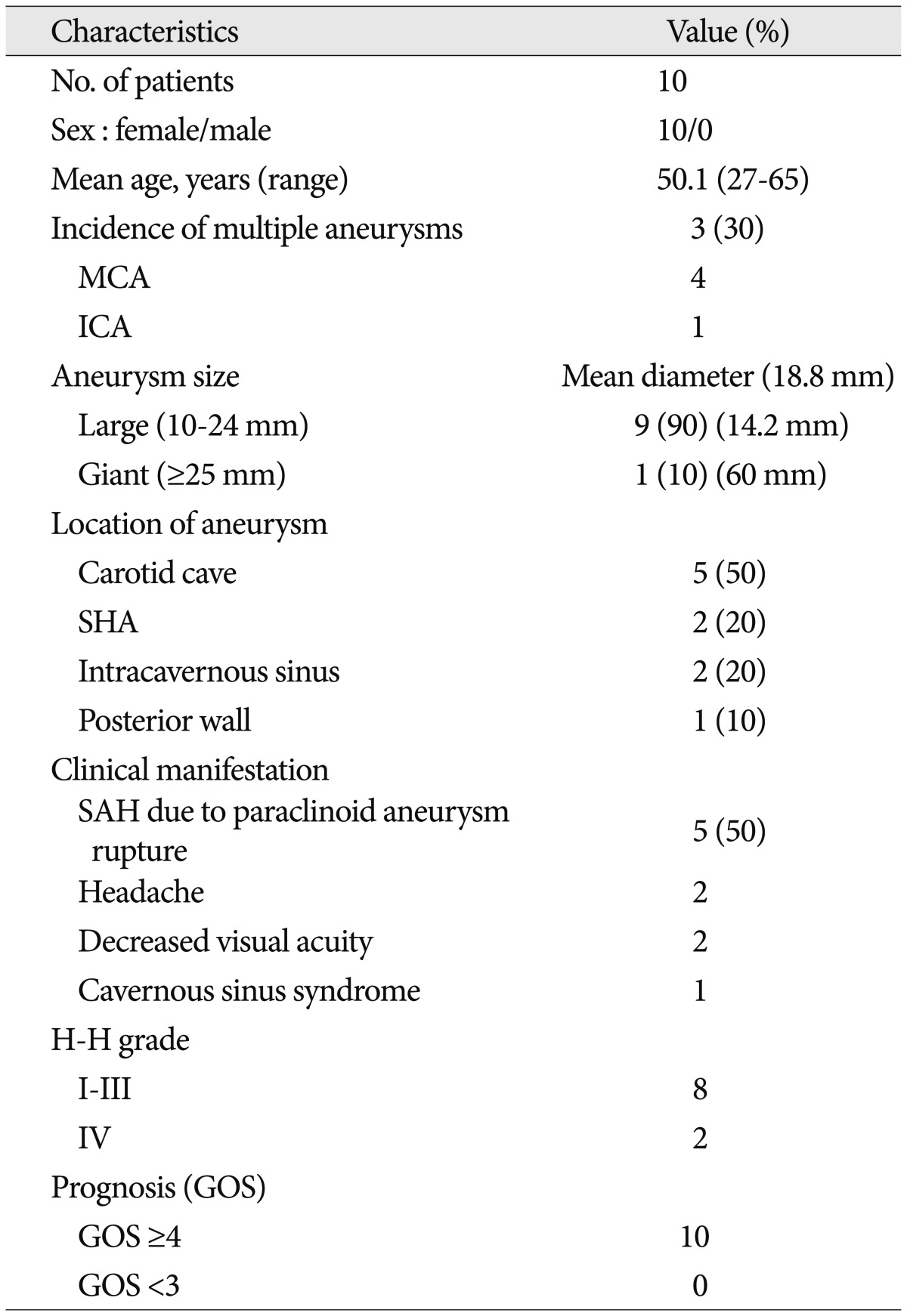
Of the ten cases reported, five each were of ruptured and unruptured aneurysms. Five aneurysms occurred in the carotid cave, two in the superior hypophyseal artery, two in the intracavernous, and one in the posterior wall. The mean diameter of the aneurysms sac was 18.8 mm in the greatest dimension. All large and giant paraclinoid aneurysms were obliterated with direct neck clipping without bypass. With the exception of the one intracavenous aneurysm, all large and giant paraclinoid aneurysms were occluded completely.
Conclusion
The key features of successful surgical clipping of large and giant paraclinoid aneurysms include enhancing exposure of proximal neck of aneurysms, establishing proximal control, and completely obliterating aneurysms with minimal manipulation of the optic nerve. Our results suggest that internal carotid artery reconstruction using multiple fenestrated clips without bypass may potentially achieve complete occlusion of large paraclinoid aneurysms.
Aneurysms arising from the proximal part of the internal carotid artery (ICA) between its exit from the proximal dural ring (oculomotor membrane) and proximal to the origin of the posterior communicating artery have been collectively termed paraclinoid aneurysms18,19,21). These paraclinoid aneurysms are classified based on complex relationships between the aneurysm sac, dura, branching arteries, and anterior clinoid process2,3,8,14,18,19,28,29). Advancements in endovascular and microsurgical techniques have substantially improved the completeness of aneurysm sac obliteration operations and the treatment outcomes of paraclinoid aneurysms5,8,9,15,16,18,30,33,35,39). However, because of the incredibly complex neuroanatomical relationship between bone, neural, and vascular structures around this paraclinoid area evidenced by high rates of surgical morbidity and mortality, direct microsurgical occlusion for large and giant paraclinoid aneurysms have remained a great challenge for vascular neurosurgeons. Additionally, in spite of recent endovascular technique improvements, large and giant aneurysms at this specific site often occlude incompletely or recur due to neck broadness33,34). Literature on the surgical results of large aneurysms in this unique anatomical area is limited5,7,9,18,22,30,32,35,39).
In this study, we reviewed our consecutive experiences in the direct surgical clipping of large and giant paraclinoid aneurysms, particularly special surgical techniques, surgery related complications, and outcomes.
A retrospective chart analysis of ten consecutive patients surgically treated for large and giant paraclinoid ICA aneurysms, performed by a single neurosurgeon between September 2001 and February 2012, was performed. All patients with large paraclinoid aneurysms were female (mean age, 50.1 years). Demographic information for each patient was gathered from medical charts, surgical videos, and pre- and postoperative images. Clinical neurological status, including postoperative complications, was collected from medical records. Initial clinical states and postoperative results were assessed by the Glasgow Coma Scale and the Glasgow Outcome Scale. All patients underwent preoperative three-dimensional (3-D) cerebral computed tomographic (CT) angiography and/or digital subtraction angiography (DSA) to determine the size, location, and exact configuration of their aneurysms. During angiography, balloon test occlusion (BTO) of the ICAs and hypotensive challenge tests were performed in five unruptured aneurysm cases in order to assess the cerebrovascular collateral reserve prior to surgery. However, only 3-D CT angiography was used in the five ruptured aneurysm cases preoperatively.
Operative strategies were planned according to the preoperative imaging studies. Cervical ICAs were exposed in all cases for proximal control. According to the aneurysm sac size, direction and the relationship between the position of proximal neck and the distal dural ring (DDR), the tailored orbito-zygomatic approach (OZA) was performed in all cases. The authors adopted the supraorbital and subtemporal modified OZAs to lower the approach related morbidity. The anterior clinoid process (ACP) was removed extradurally and intradurally with microrongeurs and 2-mm high-speed diamond burr drill (Midas High-Speed Surgical Drill®; Medtronic, Minneapolis, MN, USA). The sphenoid ridge was flattened with a high-speed diamond-burr drill or various sized micro-rongeurs until the lateral edge of the superior orbital fissure (SOF) was reached. The orbitotemporal periosteal fold (OTPF) was then peeled away from the anterior wall of the cavernous sinus (CS) and orbital apex. This maneuver was the key part of the procedure, as it facilitated dural elevation in the posteromedial direction along the lateral aspect of the ACP. Through this procedure, most of the ACP could be removed extradurally. The roof of the optic canal and most of the optic strut was removed for adequate exposure of the proximal necks of aneurysms and for intracranial proximal control of the ICA. Removal was performed intradurally with microrongeurs in most cases to avoid thermal injury of neurovascular structures. Paraclinoid aneurysms were completely obliterated via direct clipping without bypass. During the surgical procedure, effort was made to avoid optic nerve injury with minimal and brief traction of the nerve. Aneurysm sacs were decompressed and dissected using aspiration with direct puncture after proximal and distal temporary occlusion. Internal carotid artery reconstruction was performed routinely using a multiple tandem clipping technique with variously shaped fenestrated clips. Briefly, the short right angled fenestrated clip was first applied to the proximal end of the broad-neck aneurysm. The blades of fenestrated clips were applied in a parallel fashion to the ICA for reconstruction of the vessel lumen. Intraoperative Doppler was used to confirm no occlusion of perforators at the final step of operation.
The motor evoked potential (MEP), somatosensory evoked potential and visual evoked potential (VEP) were measured using an intraoperative monitoring (IOM) system (NIM-ECLIPSE system®; Medtronic, Minneapolis, MN, USA) in the five unruptured aneurysm cases. The efficacy of surgical clipping was evaluated with postoperative 3-D DSA and CT angiography in all cases. Postoperative results (i.e., clinical outcomes and surgery-related complications) were evaluated immediately following surgery, at discharge, and at the last follow-up date.
All ten patients were female (mean age, 50.1 years). Five of the ten aneurysms were unruptured, and the remaining were ruptured cases. Five aneurysms were located in the carotid cave, two in the superior hypophyseal artery, two in the CS, and one in the posterior wall. Nine of the aneurysm sacs were considered large and one was considered giant. The mean aneurysm sac diameter was 18.8 mm in the greatest dimension (Table 1). Initial clinical manifestation included bleeding (n=5), headache (n=2), decreased visual acuity (n=2), and cavernous sinus syndrome (n=1). Clinical symptoms relating to CS syndrome were caused by neural compression from large intracavernous sinus ICA aneurysm sac. The preoperative clinical neurological conditions and postoperative surgical outcomes were good in most cases (Table 1).
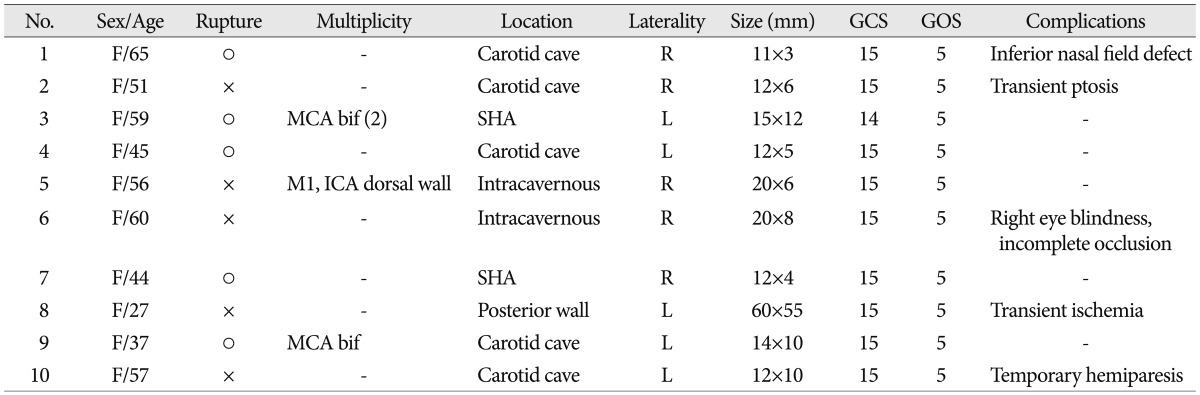
Preoperatively, all cases underwent CT angiography and/or 3-D DSA. After operation, all patients underwent CT scans to exclude surgical complications. Postoperative DSA was also performed to detect any residual aneurysm and the completeness of the aneurysm neck occlusion after clipping. Three cases had multiple aneurysms at other sites; two patients had two associated unruptured aneurysms [one had ipsilateral unruptured multiple middle cerebral artery (MCA) aneurysms, another had contralateral ICA dorsal wall and M1 aneurysms]. One patient had one contralateral unruptured MCA bifurcation aneurysm. All associated aneurysms had been also treated with surgical clipping simultaneously or through a staged operation (Table 1, 2).
With the exception of one intracavenous aneurysm, all large paraclinoid aneurysms were completely obliterated with the ICA reconstruction technique using multiple clipping with multiple fenestrated clips without bypass. Immediate surgical complications, which mainly related to ocular symptoms, occurred in five patients. Transient ptosis and hemiparesis occurred in three patients (33.3%), and recovered spontaneously. At follow-ups of at least six months, permanent visual complications had remained in two cases (20%) : one case of unilateral blindness and the other of an inferior nasal field defect on the ipsilateral side (Table 2).
A 27-year-old female presented with sudden loss of vision on the left side. Her vision had gradually deteriorated for the past two months. On neuro-ophthalmologic examination, visual acuity measured as 0.1 on the right eye by Snellen's chart, but there was total blindness on the left. There were no defects in visual fields and no limitations in extraocular movement. An aneurysm was measured just over 60 mm in diameter in its greatest dimension (Fig. 1A-D). Preoperative routine BTO was performed during preoperative DSA. Exposure of cervical ICA, intradural and extradural clinoidectomy with resection of DDR was performed routinely via OZA. The non-thrombosed giant aneurysm was directly clipped and the entire length of the supraclinoid ICA was reconstructed with eight different types of fenestrated aneurysm clips. The distal flow through the anterior cerebral artery and middle cerebral artery was nicely preserved (Fig. 1E-H). Postoperatively, the patient experienced right transient hemiparesis two days after surgery. However, it resolved two days later with hypervolemic and hypertensive therapy. Postoperatively, her right eye vision had not changed, but her left eye vision improved to a level that she could perceive hand movement 30 cm in front of her.
A 57-year-old woman presented with a one month history of blurred vision in her left eye. She had a 14-year history of hypertension for which she had been prescribed antihypertensive medication. On neuro-ophthalmologic examination, visual acuity measured as 0.9 on both sides by Snellen's chart, and no defects in visual fields or extraocular movements were noted. An unruptured left carotid cave aneurysm measuring 12×10 mm was found (Fig. 2A-D). Prior to planning surgical clipping, collateral circulation tolerance was confirmed with BTO during the preoperative DSA. The cervical ICA was exposed for proximal control and OZA with anterior clinoidectomy, and resection of the DDR was performed as part of a routine procedure to expose the proximal neck of the aneurysm. The intraoperative MEP and VEP were also monitored. During the tandem clipping and reconstruction of the aneurysm, an intraoperative rupture occurred. Ten minutes after proximal temporary occlusion of the cervical ICA, the amplitude of the MEP had completely dissipated. However, ten more minutes of proximal cervical ICA temporary occlusion was needed for complete occlusion of the broad aneurysm neck. Four differently shaped fenestrated and two standard clips were used to reconstruct the ICA with preservation of the ophthalmic and posterior communicating arteries (Fig. 2E-H). After re-opening the proximal cervical ICA temporary occlusion, the MEP amplitude had recovered to one-third of normal at the end of surgery. The patient displayed right hemiparesis (grade III) immediately after the operation, but her motor weakness gradually recovered and normalized within six hours after surgery. No other complications were noted in her last follow-up visit.
Although endovascular techniques have improved, treatment of large and giant aneurysms in the paraclinoid region remain a significant problem as they are often incompletely treated or recur because of their broad necks, recanalization, and regrowth33,34). The use of endovascular therapy in the treatment of large paraclinoid aneurysms is limited due to the superiority of the microsurgical treatment, which allows for greater durability, increased ability to ablate an associated mass effect, and decreased cost. Microsurgery of large/giant broad-neck paraclinoid aneurysms is the preferred method of treatment5,9,18,22,35).
Because of the complex topographic anatomical relationships between aneurysms arising from the proximal ICA, branching vessels, and surrounding duro-osseous structures, various terms are used for aneurysm classification1-3,8,14,18-20,28-30,38). Aneurysms in this area can be intradural, extradural, or both and there is also no reliable radiographic method to determine this12,13,25). The complex surgical anatomy, along with several factors including the size and projection of the lesion, choice of the surgical approach, relationships between the aneurysm and perforator vessels, site of proximal control, and potential improvement or worsening of visual symptoms, account for surgical difficulties5,7,9,19,22,31,35).
An accurate preoperative assessment of the origin of these aneurysms is a critical initial step in management. Having accurate microsurgical knowledge about the dural relationships of the paraclinoid region is essential to successful surgical clipping of paraclinoid aneurysms. It is also important to gain maximal exposure of the aneurysm proximal neck, establish proximal control, and completely obliterate the aneurysms with minimal manipulation of the optic nerve. First, extradural and/or intradural clinoidectomy is important and very helpful for proximal neck exposure. Additionally, those operations must be very comfortable and facile with optic strut removal, which is critical for aneurysm proximal neck exposure in most paraclinoid cases. Second, exposure of the cervical ICA is an important and useful method for proximal ICA control. Third, to avoid optic nerve injury, the pia vessels of the optic nerve must not be disrupted when incising the dura, and retraction of the optic nerve should be brief and minimal. Furthermore, the ophthalmic and superior hypophyseal arteries must be identified before incision of the distal dural ring to avoid inadvertent injury.
The data in our study differed somewhat from what has been shown previously in the literature1,2,5,7,11,15-17,26,30) in terms of sex (all females in our study), a large number of large and giant aneurysms (10/31, 32.3%), and a predominance of carotid cave aneurysms (50%). However, certain features including age (typically diverse in adults, mean age of 50.1 years in our study), a presenting symptom of bleeding (50%), and the incidence of multiple aneurysms (3/10, 30%) in our study were in line with what has been published previously1,2,5,7,9,10,15-17,22,26,30).
The complete removal of the ACP, optic canal and strut is an essential step in maximizing proximal neck exposure of large paraclinoid aneurysms and in gaining proximal control of the intracranial ICA. One drawback of the extradural anterior clinoidectomy technique is the limited extent of elevation of the frontotemporal dura over the ACP10). Bleeding from the diploic veins or the venous plexus surrounding the clinoid segment of the ICA can also make the last step of anterior clinoidectomy difficult. Several authors have recently described incision of the OTPF at the level of the lateral edge of the SOF as a key step in increasing exposure of the ACP, thus facilitating extradural clinoidectomy6,10,27,37).
Bone removal techniques using high-speed drills often result in direct mechanical or thermal injury4,36). Since having experienced a case of ipsilateral blindness in intracavernous large aneurysm clipping, we have mainly used microrongeurs or micro Kerrison punches when removing the optic canal and optic strut to avoid these surgery-related complications. Chang4) reported 45 consecutive anterior clinodectomy cases with a "no-drill" technique with no procedure-related complications in a cranial-based approach to paraclinoid and parasellar lesions. The greatest hazards relating to the surgical procedure in direct surgical clipping of large/giant paraclinoid aneurysms are microanatomical complexity and restrictions arising from limited space of intracranial proximal control, difficult bone work, broad aneurysm necks, and a lack of comprehensive anatomical knowledge, specifically of 3D contours between the aneurysm and the clinoid ICA.
Proximal control is another important issue to consider and may be achieved in the cervical carotid artery or the clinoid ICA. We always exposed the common, internal, and external carotid arteries in the neck before performing the craniotomy, and have not favored temporary balloon occlusion or suction maneuvers. We propose that exposure of the carotid artery in the neck is a much safer procedure as a means of gaining early proximal control. During surgery, exposure of the clinoid ICA and its use as a site of temporary clipping or of proximal control of an unexpected intraoperative rupture of aneurysm cannot always be readily achieved.
Special recommended clipping techniques to preserve perforators, minimize optic nerve injury, and to achieve complete obliteration of the broad neck of large/giant paraclinoid aneurysms are as follows. First, short right-angled fenestrated clips which have an aperture larger than the diameter of ICA should be used firstly. Second, the short blade, large-aperture fenestrated clips should be applied in a parallel tandem fashion to reconstruct the lumen of the ICA without damaging the branching vessels. Third, the use of multiple fenestrated clips with short blades is better than the use of a single long blade clip for reliably and completely occluding broad neck large paraclinoid aneurysms.
Five patients had transient or permanent postoperative neurological deficits (20% ruptured vs. 75% unruptured aneurysms). The common deficits were visual impairment (20%) and transient motor weakness (20%). There were no cases of operative mortality in patients harboring large/giant paraclinoid aneurysms in our series. A recent study has published an overall transient/permanent complication rate of approximately 38% in a large series of 126 surgically-treated paraclinoid aneurysms treated over 20 years, with most complications relating to visual function5). Surgery-related complications from this study were much higher in the unruptured paraclinoid aneurysm group (62.5%) than in the ruptured group (25.4%). The study also reported an operative mortality rate of 11.6% in all patients presenting with bleeding aneurysms5). Intraoperative monitoring has been only used in unruptured cases to avoid ischemic complications during temporary occlusion of the cervical or supraclinoid ICA in the dissection and direct clip application stages of operation. However, in ruptured cases, IOM was not used given the emergent conditions. The patency of parent vessels and perforators was only monitored with intraoperative Doppler. Bypass was always prepared should there be need for it during microsurgical clipping, though it was not used in our study.
Untreated aneurysms have a tendency to grow continuously and compress the optic apparatus. There are no methods of treatment leading to visual recovery or improvement30). Compared with endovascular techniques, direct obliteration of the aneurysm sac provides for mass effect reduction, offering the possibility for visual recovery. However, there are instances in which the cause of visual deterioration cannot be precisely identified.
Optic nerve injury is one of the most common complications after surgical treatment of paraclinoid aneurysms. Possible times at which optic nerve injury can occur are during the removal of the ACP and optic strut, with unroofing of the optic canal due to heat or direct injury caused by the high speed drill, and with excessive manipulation or damage to the optic nerve. There is no method which warrants the preservation of visual function. Extradural drilling of the optic canal in skull base surgery carries a potential risk of damaging the optic nerve and carotid artery4,9,24,32). Simple rongeuring of the ACP, optic canal roof, and optic strut with various shaped microrongeurs eliminates the risk of direct mechanical and thermal injury from the use of a high-speed drill instrument in a narrow surgical corridor4,32). Because the optic nerve is particularly sensitive to indirect or direct injury, minimal manipulation is most important in preserving visual function23,32). The ipsilateral total blindness seen in the patient with the large intracavernous aneurysm (case 6) may be due to a number of causes of optic nerve injury, including long segment decompression of the nerve by unpredicted mechanical injury with vibration or by thermal injury, despite copious irrigation during drilling of the optic canal roof. Intraoperative monitoring of the VEP was not helpful in predicting this permanent optic nerve injury.
ICA reconstruction using the multiple tandem clipping technique with various shaped fenestrated clips is highly valuable in most cases. Therefore, microsurgical direct clipping, under direct visualization of the aneurysm and surrounding neurovascular structures should be considered first-line treatment of large paraclinoid aneurysms. This is evidenced by the excellent long-term feasibility in complete aneurysm occlusion of the procedure, though the technique may need further study of associated morbidity.
Optic nerve injury is still the most important complication during large paraclinoid aneurysm clipping. If direct microsurgical clipping is infeasible, endovascular treatment or trapping with or without a bypass procedure, depending on the collateral flow, should be considered.
References
1. Barami K, Hernandez VS, Diaz FG, Guthikonda M. Paraclinoid Carotid Aneurysms : Surgical Management, Complications, and Outcome Based on a New Classification Scheme. Skull Base. 2003; 13:31–41. PMID: 15912157.
2. Batjer HH, Kopitnik TA, Giller CA, Samson DS. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994; 80:650–658. PMID: 8151343.

3. Beretta F. The paraclinoid aneurysms and the distal dural ring : a new classification. J Neurosurg Sci. 2004; 48:161–175. PMID: 15876985.
4. Chang DJ. The "no-drill" technique of anterior clinoidectomy : a cranial base approach to the paraclinoid and parasellar region. Neurosurgery. 2009; 64(3 Suppl):ons96–ons105. discussion ons105-ons106. PMID: 19240577.
5. Colli BO, Carlotti CG Jr, Assirati JA Jr, Abud DG, Amato MC, Dezena RA. Results of microsurgical treatment of paraclinoid carotid aneurysms. Neurosurg Rev. 2013; 36:99–114. discussion 114-115. PMID: 22898891.

6. Coscarella E, Başkaya MK, Morcos JJ. An alternative extradural exposure to the anterior clinoid process : the superior orbital fissure as a surgical corridor. Neurosurgery. 2003; 53:162–166. discussion 166-167. PMID: 12823885.
7. De Jesús O, Sekhar LN, Riedel CJ. Clinoid and paraclinoid aneurysms : surgical anatomy, operative techniques, and outcome. Surg Neurol. 1999; 51:477–487. discussion 487-488. PMID: 10321876.
8. Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985; 62:667–672. PMID: 3989589.

9. Figueiredo EG, Tavares WM, Rhoton AL Jr, De Oliveira E. Surgical nuances of giant paraclinoid aneurysms. Neurosurg Rev. 2010; 33:27–36. PMID: 19760439.

10. Froelich SC, Aziz KM, Levine NB, Theodosopoulos PV, van Loveren HR, Keller JT. Refinement of the extradural anterior clinoidectomy : surgical anatomy of the orbitotemporal periosteal fold. Neurosurgery. 2007; 61(5 Suppl 2):179–185. discussion 185-186. PMID: 18091231.
11. Fulkerson DH, Horner TG, Payner TD, Leipzig TJ, Scott JA, Denardo AJ. Endovascular retrograde suction decompression as an adjunct to surgical treatment of ophthalmic aneurysms : analysis of risks and clinical outcomes. Neurosurgery. 2009; 64(3 Suppl):ons107–ons111. discussion ons111-ons112. PMID: 19240558.
12. Gonzalez LF, Walker MT, Zabramski JM, Partovi S, Wallace RC, Spetzler RF. Distinction between paraclinoid and cavernous sinus aneurysms with computed tomographic angiography. Neurosurgery. 2003; 52:1131–1137. discussion 1138-1139. PMID: 12699558.

13. Hashimoto K, Nozaki K, Hashimoto N. Optic strut as a radiographic landmark in evaluating neck location of a paraclinoid aneurysm. Neurosurgery. 2006; 59:880–895. discussion 896-897. PMID: 17038952.

14. Heros RC, Nelson PB, Ojemann RG, Crowell RM, DeBrun G. Large and giant paraclinoid aneurysms : surgical techniques, complications, and results. Neurosurgery. 1983; 12:153–163. PMID: 6835497.

15. Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001; 48:78–89. discussion 89-90. PMID: 11152364.

16. Iihara K, Murao K, Sakai N, Shindo A, Sakai H, Higashi T, et al. Unruptured paraclinoid aneurysms : a management strategy. J Neurosurg. 2003; 99:241–247. PMID: 12924695.
17. Javalkar V, Banerjee AD, Nanda A. Paraclinoid carotid aneurysms. J Clin Neurosci. 2011; 18:13–22. PMID: 21126877.

18. Khan N, Yoshimura S, Roth P, Cesnulis E, Koenue-Leblebicioglu D, Curcic M, et al. Conventional microsurgical treatment of paraclinoid aneurysms : state of the art with the use of the selective extradural anterior clinoidectomy SEAC. Acta Neurochir Suppl. 2005; 94:23–29. PMID: 16060237.
19. Kim JM, Romano A, Sanan A, van Loveren HR, Keller JT. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery. 2000; 46:670–680. discussion 680-682. PMID: 10719864.

20. Kobayashi S, Kyoshima K, Gibo H, Hegde SA, Takemae T, Sugita K. Carotid cave aneurysms of the internal carotid artery. J Neurosurg. 1989; 70:216–221. PMID: 2913220.

21. Kolasa PP, Kaurzel Z, Lewinski A. Treatment of giant paraclinoid aneurysms. Own experience. Neuro Endocrinol Lett. 2004; 25:287–291. PMID: 15361819.
22. Li J, Lan ZG, Liu Y, He M, You C. Large and giant ventral paraclinoid carotid aneurysms : surgical techniques, complications and outcomes. Clin Neurol Neurosurg. 2012; 114:907–913. PMID: 22361473.

23. Margalit NS, Lesser JB, Moche J, Sen C. Meningiomas involving the optic nerve : technical aspects and outcomes for a series of 50 patients. Neurosurgery. 2003; 53:523–532. discussion 532-533. PMID: 12943569.
24. Mathiesen T, Kihlström L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006; 59:570–576. discussion 570-576. PMID: 16955039.

25. Murayama Y, Sakurama K, Satoh K, Nagahiro S. Identification of the carotid artery dural ring by using three-dimensional computerized tomography angiography. Technical note. J Neurosurg. 2001; 95:533–536. PMID: 11565882.

26. Nanda A, Javalkar V. Microneurosurgical management of ophthalmic segment of the internal carotid artery aneurysms : single-surgeon operative experience from Louisiana State University, Shreveport. Neurosurgery. 2011; 68:355–370. discussion 370-371. PMID: 21135716.
27. Noguchi A, Balasingam V, Shiokawa Y, McMenomey SO, Delashaw JB Jr. Extradural anterior clinoidectomy. Technical note. J Neurosurg. 2005; 102:945–950. PMID: 15926728.
28. Nutik SL. Ventral paraclinoid carotid aneurysms. J Neurosurg. 1988; 69:340–344. PMID: 3404229.
29. Pia HW. Classification of aneurysms of the internal carotid system. Acta Neurochir (Wien). 1978; 40:5–31. PMID: 654969.

30. Raco A, Frati A, Santoro A, Vangelista T, Salvati M, Delfini R, et al. Long-term surgical results with aneurysms involving the ophthalmic segment of the carotid artery. J Neurosurg. 2008; 108:1200–1210. PMID: 18518728.

32. Son HE, Park MS, Kim SM, Jung SS, Park KS, Chung SY. The avoidance of microsurgical complications in the extradural anterior clinoidectomy to paraclinoid aneurysms. J Korean Neurosurg Soc. 2010; 48:199–206. PMID: 21082045.

33. Thornton J, Aletich VA, Debrun GM, Alazzaz A, Misra M, Charbel F, et al. Endovascular treatment of paraclinoid aneurysms. Surg Neurol. 2000; 54:288–299. PMID: 11136984.

34. Wang Y, Li Y, Jiang C, Jiang F, Meng H, Siddiqui AH, et al. Endovascular treatment of paraclinoid aneurysms : 142 aneurysms in one centre. J Neurointerv Surg. 2013; 5:552–556. PMID: 23087381.

35. Xu BN, Sun ZH, Romani R, Jiang JL, Wu C, Zhou DB, et al. Microsurgical management of large and giant paraclinoid aneurysms. World Neurosurg. 2010; 73:137–146. discussion e17, e19. PMID: 20860951.

36. Xu D, Pollock M. Experimental nerve thermal injury. Brain. 1994; 117(Pt 2):375–384. PMID: 8186963.

37. Yang Y, Wang H, Shao Y, Wei Z, Zhu S, Wang J. Extradural anterior clinoidectomy as an alternative approach for optic nerve decompression : anatomic study and clinical experience. Neurosurgery. 2006; 59(4 Suppl 2):ONS253–ONS262. PMID: 17041495.
38. Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975; 3:7–14. PMID: 1111150.
39. Zipfel GJ, Day AL. Surgical treatment of clinoid and ophthalmic segment internal carotid artery aneurysms. In : Roux PD, Winn HR, Newell DW, editors. Management of cerebral aneurysms. Philadelphia: Saunders;2004. p. 731–744.
Fig. 1
Preoperative left digital subtraction angiography (DSA) (A, B and C) demonstrating a giant (60×55 mm sized sac) left internal carotid artery (ICA) posterior wall aneurysm. Contrast media mainly accumulated due to rapid shunting flow into the giant non-thrombosed aneurysm sac, resulting in poor visualization of the proximal and distal ICA flow. A : Anteroposterior view. B : lateral view. C : anterooblique view of three-dimensional (3-D) DSA. Preoperative 3-D computed tomographic (CT) angiography (D) showing a giant sac adheres to all of the surrounding anterior and posterior cerebral arteries on both sides. Postoperative left 3-D DSA (E, F and G) demonstrating complete obliteration of a giant ICA posterior wall aneurysm sac and the entire reconstructed length of ICA with preservation of ophthalmic artery using a clipping technique of eight different shapes of fenestrated clips. E : Anteroposterior view. F : lateral view. G : lateral view of 3-D DSA. Postoperative 3-D CT angiography (H) showing the preservation of the left anterior and middle cerebral vascular trees.
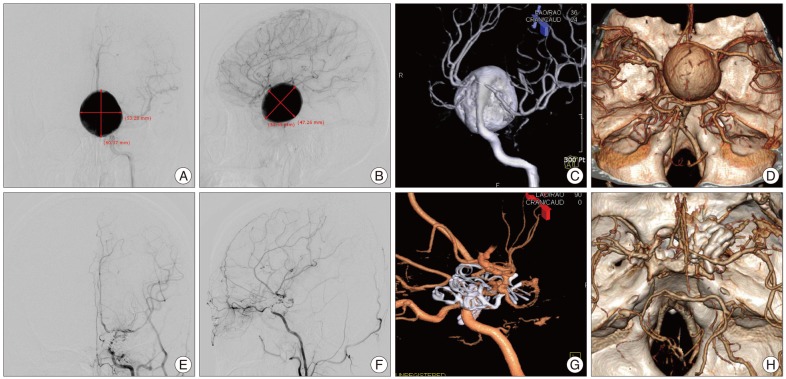
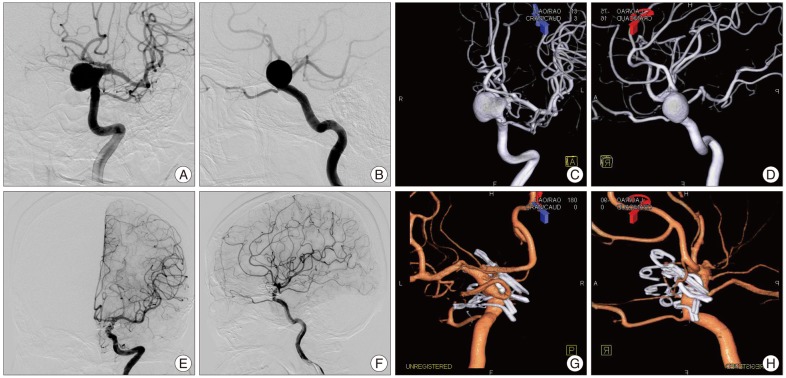
Fig. 2
Preoperative left carotid digital subtraction angiography (DSA) (A-D) demonstrating a large (12×10 mm sized sac) left carotid cave aneurysm. A : Anteroposterior view. B : lateral view. C : anteroposterior view of three-dimensional (3-D) DSA. D : lateral view of 3-D DSA. The ophthalmic and posterior communicating arteries are closely related with the aneurysm. Postoperative left carotid DSA (E-H) demonstrating a complete occlusion of a large carotid cave aneurysm sac and reconstructed proximal internal carotid artery with sparing of the left ophthalmic and posterior communicating arteries using six various types of aneurysm clips. E : Anteroposterior view. F : lateral view. G : medial view of 3-D DSA. H : mediolateral view of 3-D DSA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download