Abstract
Objective
Density of the chronic subdural hematoma (cSDH) is variable. It often appears to be mixed density. Multiple densities of cSDH may result from multiple episodes of trauma. We investigated the frequency of mixed density and the causes of head injuries representing each density.
Methods
We could collect 242 cases of chronic SDH. The cSDHs were classified into four groups; hypodensity, homogeneous isodensity, layered type, and mixed type on the basis of CT scans.
Results
The density of cSDH was isodense in 115 patients, hypodense in 31 patients, mixed in 79 cases, and layered in 17 cases. The cSDH was on the left side in 115 patients, on the right side in 70 patients, and bilateral in 40 patients. The history of trauma was identifiable in 122 patients. The etiology could be identified in 67.7% of the hypodense hematomas, while it was obscure in 59.5% of the mixed hematomas.
Conclusion
Mixed density of cSDH results from multiple episodes of trauma, usually in the aged. It is hard to remember all the trivial traumas for the patients with the mixed density cSDHs. Although there were membranes within the mixed density hematomas, burr-holes were usually enough to drain the hematomas.
Computed tomography (CT) remains the preferred diagnostic method for the chronic subdural hematoma (cSDH)3). Chronic SDH has a variety of imaging characteristics in CT; low, intermediate, or high density relative to brain parenchyma3,6). Chronic SDH frequently appears to be mixed density7,9). With current high-resolution CT scanners, homogeneous isodensity becomes rare21). Acute trauma on the patients with cSDH may develop acute bleeding over the cSDH, which would produce mixed density10). Repeated episodes of acute bleeding may result mixed densities of SDH. In other words, mixed density suggests multiple episodes of trauma. We investigated the frequency of mixed density in cSDH. We also studied the cause of trauma representing each density of cSDH.
We retrospectively examined the medical records and CT scans of 259 consecutive patients who diagnosed as cSDH from January 2006 to December 2011. We excluded 17 patients who diagnosed by magnetic resonance imaging (MRI) only before surgery. The male/female ratio was 186/56.
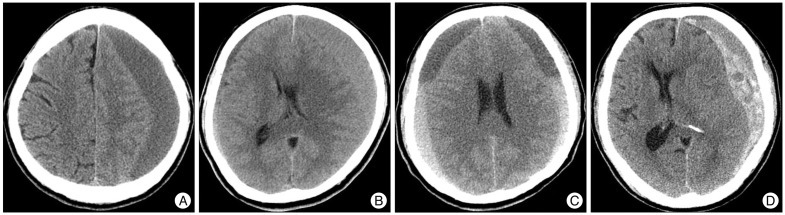
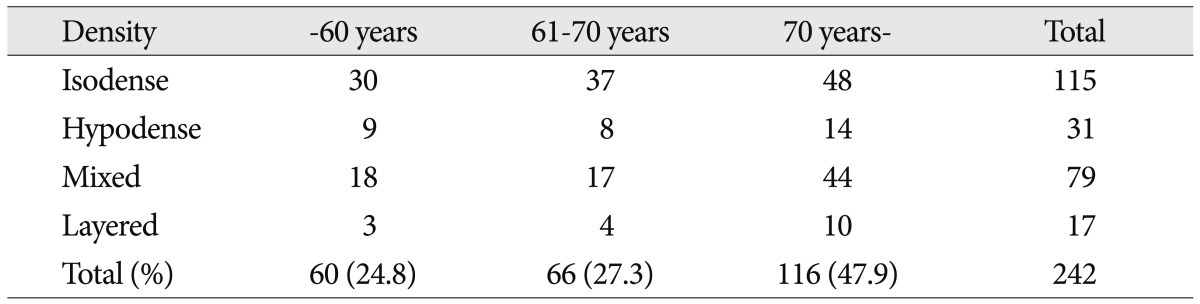
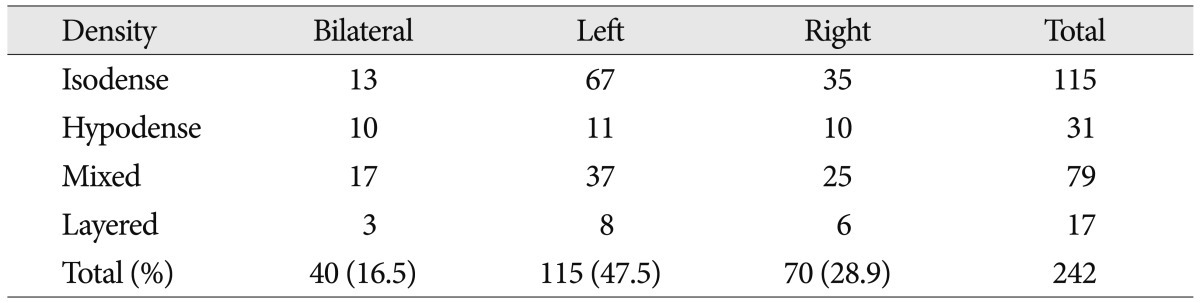
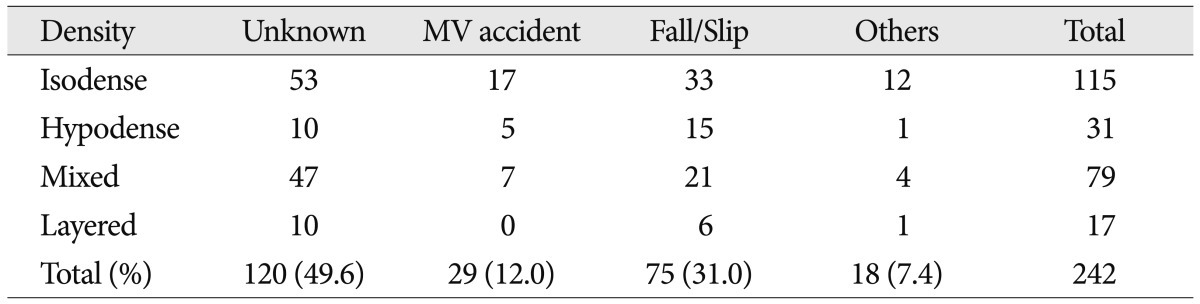
The cSDHs were classified into four groups; hypodensity (<25 HU), homogeneous isodensity (25-35 HU), layered type, and mixed type on the basis of CT scans (Fig. 1).
Although we examined the history of head trauma minutely, the etiology was identifiable in only 122 patients (50.4%). Statistical analysis was performed using the chi-square test or Fisher's exact test. For the statistical significance, we divided the etiology into either known or unknown groups. Differences were considered significant if the probability value was less than 0.05.
The density of cSDH was isodense in 115 patients, hypodense in 31 patients, mixed in 79 cases, and layered in 17 cases (Table 1). Mixed or layered types were more common in the oldest age, while isodense or hypodense SDHs were more common the age of less than 70 years (p=0.0002 by Fisher).
The cSDH was on the left side in 115 patients, on the right side in 70 patients, and bilateral 40 patients (Table 2). Bilateral hematomas tended to be hypodense, while isodense one tended to locate on the left side (p=0.015 by Fisher).
We identified the causes of head trauma in 122 patients (50.4%). Slipping was the most common cause of head trauma (Table 3). Motor vehicle accidents or falling was also relatively common known cause. The etiology could be identified in 67.7% of the hypodense hematomas, while the cause was obscure in 59.5% of the mixed hematomas (p=0.047 by chi-square).
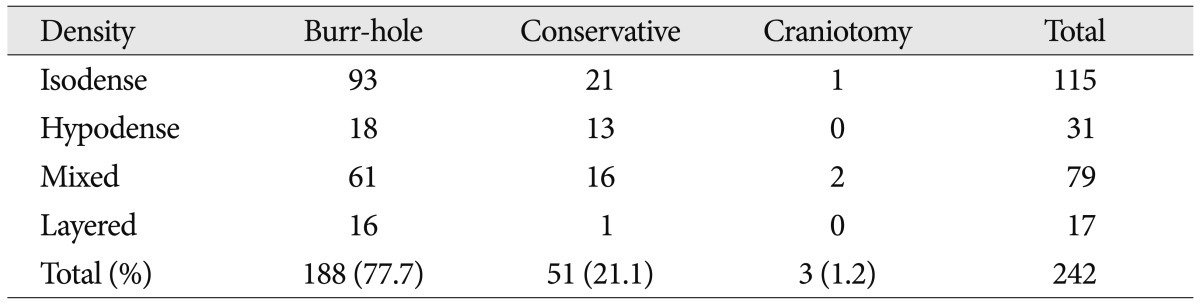
The preferred surgical technique was single burr-hole drainage under local anesthesia (Table 4). Conservative treatment was used in more than 40% of the patients with hypodense hematomas. We performed craniotomy for 2 patients with mixed density hematomas and one patient with isodense hematomas. We removed some clot with gentle irrigation and suction.
In one patient, we used an endoscopy to suck out the semisolid clot around the corner of the hematoma cavity. The method of treatment differed among the hematoma densities (p=0.017 by Fisher).
Isodensity was the most common density of cSDHs. There are some reports that the hypodensity was the most common type in CT scanning3,6). However, isodense cSDHs were often reported as more common than the hypodense lesions1,5,7,9). It may depend on the patient population, resolution of the CT scanner, and methods of density classification. Mixed density was relatively common, being roughly one third of cases in this study. It shared from 16% to 63% of cSDHs1,6,7,17).
A layered type of the hematoma density may result from prolonged recumbency, which separates the blood components and fluid8,20). The CT density coefficients reflect the blood components. If the patient maintains lying position for a long time, more heavier components sink by the gravity. Elderly people are more likely to stay lying down for a long period of declining physical activity. The blood components are separated by gravity and layered density is appeared in the CT scan.
Theoretically, the mixed density results from three hypotheses. The first situation is in hyper-acute bleeding; the difference between solid clot and liquid blood may produce the mixed density15). The second situation is in subacute SDHs; resolving hematoma may appear peripheral hypodensity with central hyperdensity during transitional period9). The reason is the local difference between the hemolytic activity. Fibrinolysis in CSF is activated from the outside to the inside in order after trauma. The change into the hypodensity is faster in the peripheral region than in the center, where the density of hematoma remains hyperdense for a long time. The CT density can appear to be mixed density during this transitional period. However, these two situations are relatively short to get the mixed density images in the CT scans. It is well known that the repeated microhemorrhages were responsible for the enlargement of cSDH2,11). The bleeding from the sinusoidal vessels of the outer neomembrane makes the hematoma grow without coagulation13). Insidious rebleeding is hard to produce mixed density instead of raising the density. Even though the cSDH continue to enlarge, it may remain asymptomatic, when the reserving capacity was remained or well balanced. The hematoma pressure was 15 cmH2O or below in a half of unilateral cSDHs, especially in the elderly18). Although some patients with cSDH are still asymptomatic, they are prone to fall or slip down. If they slip, even though the injury itself is trivial, it may tear the cortical bridge veins or fragile vessels in the neomembrane. Repeated trauma may cause acute bleeding, which would make a lump or a layer of hyperdensity within hypo- or isodense hematoma. Like the repeated microhemorrhages from the outer membrane, repeated trauma may cause acute bleeding over the cSDH as a mechanism of hematoma enlargement10). Sometimes repeated trivial trauma may cause a subdural hygroma, which became a cSDH. Although the ages of the SDHs were different, such a chronic-on-chronic SDH may produce the mixed density.
MRI is superior to CT when detecting membranes in cSDHs2,16,19). Membranes were frequently observed within the SDHs, especially in hematomas with the mixed density2,16). The membranes appeared either multi-lobule or multi-layer. The acute-on-chronic SDHs tend to produce so-called multi-lobule type membrane, while chronic-on-chronic SDHs would produce multi-layer type membrane.
Mixed or layered types were more common in the oldest age, while isodense or hypodense SDHs were more common the age of less than 70 years. This uneven distribution result from not only the fact that the reserving capacity is maximal in the oldest age, but also the oldest patients are vulnerable to repeated trauma. Also, elderly patients cannot remember their trivial trauma events because of cognitive impairment. The oldest age implies brain atrophy and too high reserving capacity, which may cause overlapping hematomas or recurrence. Multilayer intra-hematomal structure showed a high recurrence rate19).
Bilateral hematomas tended to be hypodense, while isodense one tended to locate on the left side, in this study. The reason is the cSDH originated from SDGs would be bilateral and hypodense4). Cranial asymmetry is also the cause of the left prevalence of cSDH.
It is hard to obtain the exact cause of trauma in cSDHs. A definite history of head injury was often obtained only in a half of cases, and more than 90% of previous head injuries were mild12). We could identify the causes of head trauma in less than a half. The etiology was obscure especially in the mixed hematomas. The reason is that the multiple episodes of trivial trauma are hard to remember. Considering the facts that mixed types were more common in elderly patients and the cause of trauma was obscure in the mixed hematomas, we could deduce mixed types occurred by multiple trivial trauma in elderly people.
There are many conservative or surgical treatment methods for the management of cSDH14). Burr-hole drainage is sufficient for most patients and this became the procedure of choice7,11,14). We could remove the hematoma by single or two burr-holes in most cases. For the bilateral cSDHs, we used bilateral single burr-hole drainage. We used double burr-holes with saline irrigation in only a few patients. In patients with mixed density cSDH, often the hematomas were a mixture of semisolid clot and liquefied hematoma. Even in acute-on-chronic SDHs the clot was not so hard due to preexisting hemolytic activity of the cSDH. We did not try to remove the clot vigorously. We placed a soft silicon drain in all cases, which was usually removed within 2 days. Semisolid clot was usually drained out or resolved within a few days with or without urokinase or tissue plasminogen activator. Septation within the hematoma was usually not complete in either multi-lobule or multi-layer hematomas. There was free communication, which allows draining out the hematomas. Craniotomy may be necessary for those instances in which the subdural collection reaccumulates, the brain fails to expand, or there is solid hematoma11).
Mixed density of cSDH was relatively common, being 33% in this study. Mixed density of cSDH results from multiple episodes of trauma, especially in the oldest age. The etiology was frequently obscure in the mixed density cSDHs, since it was hard to remember all the trivial traumas. Although there were membranes within the hematoma, burr-hole was usually enough to drain the hematomas.
References
1. Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2012; 154:1541–1548. PMID: 22653496.

2. Fujisawa H, Nomura S, Kajiwara K, Kato S, Fujii M, Suzuki M. Various magnetic resonance imaging patterns of chronic subdural hematomas : indicators of the pathogenesis? Neurol Med Chir (Tokyo). 2006; 46:333–338. discussion 338-339. PMID: 16861826.
3. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma : surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005; 107:223–229. PMID: 15823679.

4. Kim BG, Lee KS, Shim JJ, Yoon SM, Doh JW, Bae HG. What determines the laterality of the chronic subdural hematoma? J Korean Neurosurg Soc. 2010; 47:424–427. PMID: 20617086.

5. Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008; 43:11–15. PMID: 19096538.

6. Kostanian V, Choi JC, Liker MA, Go JL, Zee CS. Computed tomographic characteristics of chronic subdural hematomas. Neurosurg Clin N Am. 2000; 11:479–489. PMID: 10918018.

7. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients : is membranectomy necessary? Surg Neurol. 2004; 61:523–527. discussion 527-528. PMID: 15165784.

8. Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004; 18:351–358. PMID: 14742149.
9. Lee KS, Bae WK, Bae HG, Doh JW, Yun IG. The computed tomographic attenuation and the age of subdural hematomas. J Korean Med Sci. 1997; 12:353–359. PMID: 9288636.

10. Lee KS, Shim JJ, Yoon SM, Doh JW, Yun IG, Bae HG. Acute-on-chronic subdural hematoma : not uncommon events. J Korean Neurosurg Soc. 2011; 50:512–516. PMID: 22323938.

11. Markwalder TM. Chronic subdural hematomas : a review. J Neurosurg. 1981; 54:637–645. PMID: 7014792.
12. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases : clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381. PMID: 11561347.

13. Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al. Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role of thrombomodulin in the mechanism. J Neurosurg. 2002; 96:877–884. PMID: 12005395.

14. Santarius T, Hutchinson PJ. Chronic subdural haematoma : time to rationalize treatment? Br J Neurosurg. 2004; 18:328–332. PMID: 15702829.
15. Sargent S, Kennedy JG, Kaplan JA. "Hyperacute" subdural hematoma : CT mimic of recurrent episodes of bleeding in the setting of child abuse. J Forensic Sci. 1996; 41:314–316. PMID: 8871392.
16. Senturk S, Guzel A, Bilici A, Takmaz I, Guzel E, Aluclu MU, et al. CT and MR imaging of chronic subdural hematomas : a comparative study. Swiss Med Wkly. 2010; 140:335–340. PMID: 20349366.
17. Stroobandt G, Fransen P, Thauvoy C, Menard E. Pathogenetic factors in chronic subdural haematoma and causes of recurrence after drainage. Acta Neurochir (Wien). 1995; 137:6–14. PMID: 8748860.

18. Sundstrøm T, Helland CA, Aarhus M, Wester K. What is the pressure in chronic subdural hematomas? A prospective, population-based study. J Neurotrauma. 2012; 29:137–142. PMID: 21635185.

19. Tanikawa M, Mase M, Yamada K, Yamashita N, Matsumoto T, Banno T, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien). 2001; 143:613–618. discussion 618-619. PMID: 11534679.

20. Tsai FY, Huprich JE, Segall HD, Teal JS. The contrast-enhanced CT scan in the diagnosis of isodense subdural hematoma. J Neurosurg. 1979; 50:64–69. PMID: 758381.

21. Wilms G, Marchal G, Geusens E, Raaijmakers C, Van Calenbergh F, Goffin J, et al. Isodense subdural haematomas on CT : MRI findings. Neuroradiology. 1992; 34:497–499. PMID: 1436458.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download