Abstract
Split cord malformations (SCMs) usually present in childhood, and are rarely reported in adults. And also, a cervicothoracic SCM associated with tethered cord syndrome has very rarely been reported in the literature. We report a case of SCM associated with tethered cord and spina bifida in an adult. This report describes the case of a 34-year-old woman who presented for evaluation of neck pain, back pain, and intermittent paraparesis of several months duration. The MRI and CT showed a SCM at the cervicothoracic level and a fibrous septum at the thoracic level. She underwent surgery for the SCM and tethered cord syndrome, and was followed for 7 years. Patient presented complete recovery in the follow-up. The authors discuss this unusual lesion and describe the anatomical relationship of the level of cord duplication and fibrous septum.
Split cord malformations (SCMs) are rare congenital anomalies in which the cord is split over a portion of its length to form a double neural tube in a single dural sac2,5,16,20).
SCMs associated with a split of the spinal column, spinal bony spurs, myeloceles, myelomeningoceles, lipomas, and dermal sinuses have been reported in the literature10,12,13,22).
SCMs and other associated anomalies are rare within the general population. It is more uncommon for the initial clinical presentations to occur in adulthood4).
We report a rare case of cervicothoracic SCM with spina bifida and tethered cord syndrome due to the thickened filum terminale in adult and discuss the anatomical and embryological relationship of the level of the fibrous septum and duplicated level of the spinal cord.
A 34-year-old woman was admitted with neck pain, back pain, and recurrent bilateral leg weakness of 2 months duration. On physical examination, there was a paresis with 4/5 muscle strength and deep tendon hyperreflexia in both legs. There were no pathologic reflexes. The initial neurologic evaluation revealed sensory numbness below the level of the C-7 dermatome. An urodynamic study, somatosensory evoked potentials, and electromyograhic evaluation showed no abnormalities.
The radiologic examination revealed mild scoliosis of the thoracic spine, hemi-vertebrae at T6 level, and butterfly vertebral bodies at T11 level. Spina bifida was also present. The admission cervicothoracic MRI showed that the spinal cord was divided into two segments from the level of C-7 to T-11, and there was development the subcutaneous fat within the bifid spine. No fibrous septum was demonstrated on MR imaging. On lumbosacral MRI, the conus was shown to be low-lying, ending at the L5 level (Fig. 1). CT myelography showed that dye was leaked incidentally into the epidural and intradural spaces. A fibrous septum divided the spinal cord and extended to the epidural space and attached to the ventral cortex of the lamina at T9 level (Fig. 2).
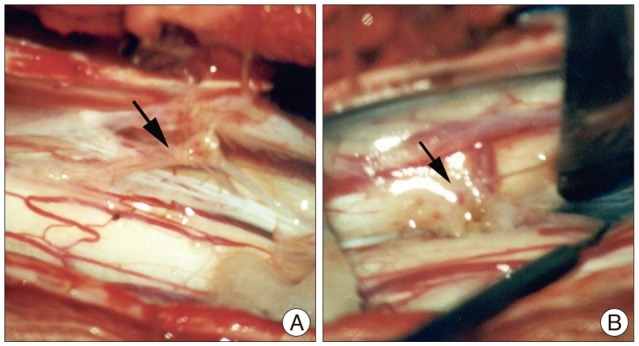
The patient underwent surgery to prevent progression of the neurologic damage resulting from tethering of the spinal cord. A laminectomy was performed from the level of T-8 to T-11. The T-9 and T-10 spines were bifid and abnormal fat tissue was infiltrated between the bisected spinous process. Surgery revealed a symmetric duplication of the spinal cord above the level of T-10, which was encompassed by a single dural sac, and the fibrous septum was recognized between the divided hemi-cord at the T-9 level, 10 mm above the cord reunion site (T-10). The fibrous septum splits and tethers spinal cord. The tethered spinal cord was released by resection of the fibrous septum (Fig. 3).
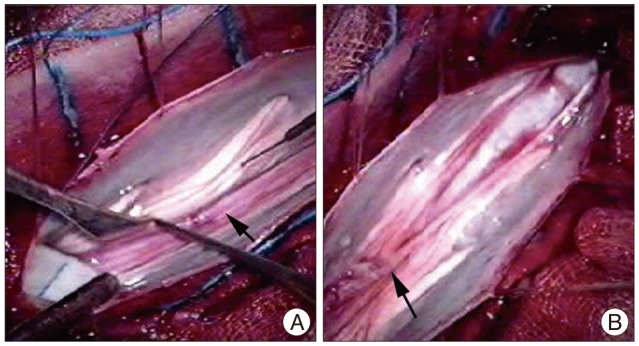
Afterwards, a L5 and S1 laminectomy was performed to release the tethered cord syndrome by the thickened filum terminale21). The nerve roots appeared slightly adherent to the dura at the L-5 level, and resection of the thickened filum was made because the cord was tethered at the level of the lesion (Fig. 4). The patient had an uneventful postsurgical recovery.
SCM is a rare spinal anomaly that refers to a longitudinal division of the spinal cord into two divided hemi-cords6,11). This malformation has been reported to be associated with a split of the spinal column, spinal bony spurs, myeloceles, myelomeningoceles, lipomas, and dermal sinus15,16).
SCMs are often located in the lumbar and thoracolumbar regions. Sinha et al. reported the incidence rates for cervical and cervicothoracic locations as 3% and 1%, respectively21).
Symptoms related to congenital anomalies occur most commonly in childhood, so SCMs were initially regarded as a pediatric problem; but in many patients the diagnosis is not established until symptoms manifest in adulthood1,9,18,19).
Embryologically, the spinal cord is formed by the integration of two paramedian notochords along the midline17). When this integration is defective, a SCM can develop.
SCMs are classified as one of two types, according to the unified theory : in type I SCM, the hemi-cords are always invested with individual dural sacs and the medial walls of the sacs always ensheath a rigid (bony or cartilaginous) midline spur; and in type II SCM the hemi-cords are always within a single dural sac and the midline septum is always composed of non-rigid fibrous or fibrovascular tissues16,17). Based on this classification, our case can be classified as type II because the spinal cord was divided by a fibrous septum at the level of T-9.
An interesting finding of this case was the relationship between the anatomic location of the fibrous septum and the level of duplication of the SCM. Embryologically, SCM is caused by a primary neurulation defect. According to the unified theory of embryogenesis proposed by Pang et al.17), an abnormal communication (endomesenchymal tract) between the ectoderm and endoderm causes regional splitting of the notochord, and each separated notochord induces the surrounding paraxial mesoderm and subsequent dysgenesis of the spinal cord. Thus, the level of the fibrous septum and duplicated level of the spinal cord should be similar. However, the fibrous septum was located farther above the level of the spinal cord duplication in this case. It might be because this case was associated with a thickened filum terminale. A low-lying conus and thick filum terminale are related to abnormal retrogressive differentiation of the secondary neural tube with failure of terminal cord involution7,17). The thickened filum terminale fixed conus and relative ascent of the spinal cord was not complete. This embryologic background could be the basis for the fibrous septum located proximal to the level of the spinal cord duplication in this case. And also, anatomical location of the fibrous septum and level of cord duplication of this case could delineate the sequence of spinal cord development.
This anatomic relationship might be helpful to identify the fibrous septum to detether type II SCM patients associated with thick filum terminale. Associated skeletal dysgenesis such as spina bifida, hemi-vertebrae, or a laminar defect might also be helpful to decide the level of detethering surgery.
An awareness of the presence of associated congenital anomalies is surgically important to improve postoperative outcomes and to determine surgical priorities3,8,22) For example, if the SCM is accompanied by a thickened filum terminale, the septum should first be excised to release the cord. Otherwise, the abruptly released cord will suddenly be pulled upwards, resulting in spinal cord injury below the level of the SCM14).
Unlike pediatric patients, adult-onset SCM may present without neurocutaneous or overt skeletal abnormalities. A thorough evaluation of associated anomalies is mandatory to make a therapeutic plan for patients with SCM. Although it is rare, careful preoperative examination and refined microsurgery may provide good outcome for the patient.
References
1. Akay KM, Izci Y, Baysefer A, Timurkaynak E. Split cord malformation in adults. Neurosurg Rev. 2004; 27:99–105. PMID: 14618409.

2. Anderson RC, Ragel BT, Mocco J, Bohman LE, Brockmeyer DL. Selection of a rigid internal fixation construct for stabilization at the craniovertebral junction in pediatric patients. J Neurosurg. 2007; 107:36–42. PMID: 17644919.

3. Andro C, Pecquery R, De Vries P, Forlodou P, Fenoll B. Split cervical spinal cord malformation and vertebral dysgenesis. Orthop Traumatol Surg Res. 2009; 95:547–550. PMID: 19837020.

4. Chehrazi B, Haldeman S. Adult onset of tethered spinal cord syndrome due to fibrous diastematomyelia : case report. Neurosurgery. 1985; 16:681–685. PMID: 3889701.

6. Guilloton L, Allary M, Jacquin O, Billaud Y, Drouet A, Felten D, et al. [Split-cord malformation (diastematolmyelia) presenting in two adults case report and a review of the literature]. Rev Neurol (Paris). 2004; 160:1180–1186. PMID: 15602364.
7. Hertzler DA 2nd, DePowell JJ, Stevenson CB, Mangano FT. Tethered cord syndrome : a review of the literature from embryology to adult presentation. Neurosurg Focus. 2010; 29:E1. PMID: 20593997.
8. Jindal A, Mahapatra AK. Split cord malformations--a clinical study of 48 cases. Indian Pediatr. 2000; 37:603–607. PMID: 10869139.
9. Kaminker R, Fabry J, Midha R, Finkelstein JA. Split cord malformation with diastematomyelia presenting as neurogenic claudication in an adult : a case report. Spine (Phila Pa 1976). 2000; 25:2269–2271. PMID: 10973414.

10. Lapsiwala SB, Iskandar BJ. The tethered cord syndrome in adults with spina bifida occulta. Neurol Res. 2004; 26:735–740. PMID: 15494114.

11. Lewandrowski KU, Rachlin JR, Glazer PA. Diastematomyelia presenting as progressive weakness in an adult after spinal fusion for adolescent idiopathic scoliosis. Spine J. 2004; 4:116–119. PMID: 14749200.

12. Mahapatra AK, Gupta DK. Split cord malformations: a clinical study of 254 patients and a proposal for a new clinical-imaging classification. J Neurosurg. 2005; 103:531–536. PMID: 16383252.

13. Muroi A, Fleming KL, McComb JG. Split medulla in association with multiple closed neural tube defects. Childs Nerv Syst. 2010; 26:967–971. PMID: 20179945.

14. Ozturk E, Sonmez G, Mutlu H, Sildiroglu HO, Velioglu M, Basekim CC, et al. Split-cord malformation and accompanying anomalies. J Neuroradiol. 2008; 35:150–156. PMID: 18206241.

15. Pallatroni HF, Ball PA, Duhaime AC. Split cord malformation as a cause of tethered cord syndrome in a 78-year-old female. Pediatr Neurosurg. 2004; 40:80–83. PMID: 15292638.

16. Pang D. Split cord malformation : Part II : Clinical syndrome. Neurosurgery. 1992; 31:481–500. PMID: 1407429.
17. Pang D, Dias MS, Ahab-Barmada M. Split cord malformation : Part I : A unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992; 31:451–480. PMID: 1407428.
18. Peart O. Split cord malformation. Radiol Technol. 2004; 76:168. PMID: 15587620.
19. Sato K, Yoshida Y, Shirane R, Yoshimoto T. A split cord malformation with paresis of the unilateral lower limb : case report. Surg Neurol. 2002; 58:406–409. discussion 409. PMID: 12517623.

20. Sharma A. Split cord malformations. Indian Pediatr. 2001; 38:1069–1070. PMID: 11568396.
21. Wehby MC, O'Hollaren PS, Abtin K, Hume JL, Richards BJ. Occult tight filum terminale syndrome : results of surgical untethering. Pediatr Neurosurg. 2004; 40:51–57. discussion 58. PMID: 15292632.

22. Yamada S, Colohan AR, Won DJ. Tethered cord syndrome. J Neurosurg Spine. 2009; 10:79–80. author reply 80-81. PMID: 19119938.

Fig. 1
Plain radiograph of thoracic spine reveals scoliosis at the cervicothoracic junction, hemi-vertebrae (arrowhead), and spina bifida (white arrow). B : Coronal section MR image shows the evidence of clefting of the cervicothoracic spinal cord and vertebral dysgenesis. C : Axial T2-weighted image shows the spinal cord is split into two hemi-cords within the single dural sac at the thoracic level. Common midline arachnoid space is identified between the two hemi-cords. Abnormal fat tissue accumulation is noted between the bifid spinous processes (arrow). D : Sagittal T2-weighted MR image shows the low-lying conus medullaris tethered posteriorly in the spinal canal.
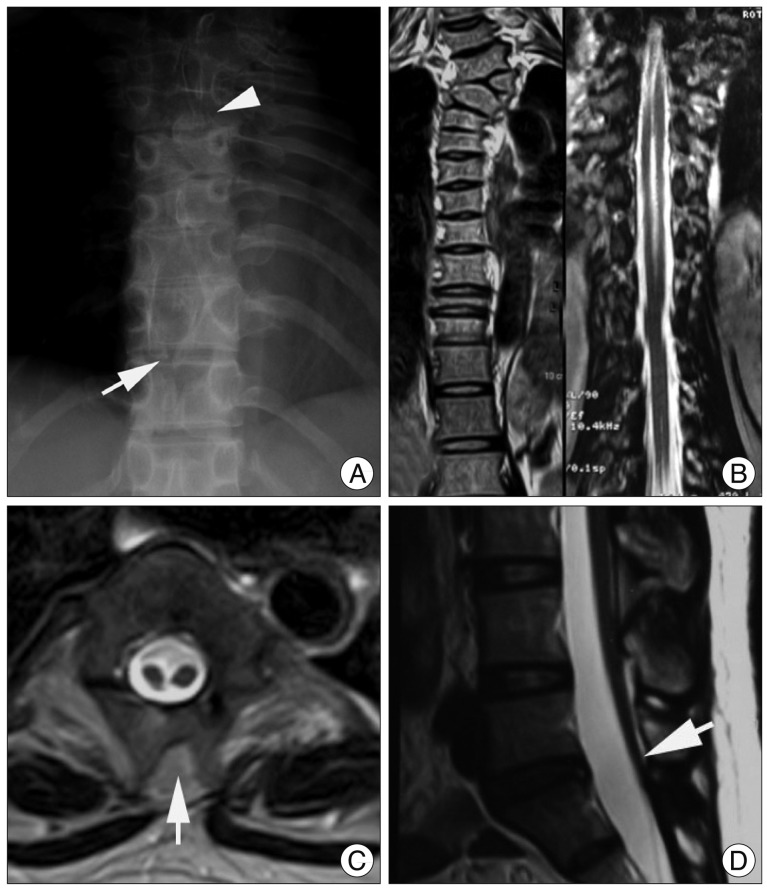
Fig. 2
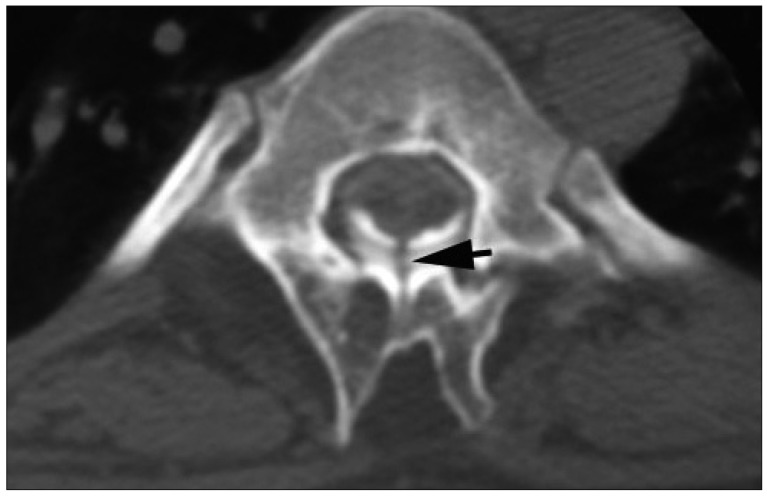
Axial CT myelography shows the presence of fibrous septum which extends to the epidural space and is attached into the lamina.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download