Abstract
Objective
The purposes of this study were to evaluate the prevalence, types, and locations of Modic changes (MCs) in the thoracic spine in a large number of subjects, and to investigate the relation between the distributions of MCs and disc herniations (DHs) in the thoracic spine.
Methods
Two experienced musculoskeletal radiologists assessed the presence of MCs and DHs by consensus in the thoracic MRIs of 144 patients with non-specific back pain. Patient ages ranged from 22 to 88 years (mean=53.3±14.66 years), and 72 were female (50%). The prevalence, distribution, relation of MCs and DHs was recorded.
Results
MC was observed in 8 of the 144 patients (5.6%) and 10 of 1728 segments (0.58%). The most common MC was type II. Of the 8 patients exhibiting MC, 6 had type II (75.0%), and 2 had mixed MCs (type I/II or type II/III). MCs were distributed mainly at the mid-thoracic level (from T5/6 to T9/10). DH was detected in 18 patients (12.5%), 36 of 1728 segments (2.1%). Of the 10 segments exhibiting MC, 5 had DHs at the same level (50.0%). Accordingly, DH was strongly associated with MC (p=0.000).
Conclusion
A low prevalence of MC was observed in the thoracic spine, and type II MC predominated. The low prevalence of MC in the thoracic spine suggests that it was caused by a relative lack of mobility as compared with the cervical and lumbar spines. And DHs were found to be strongly associated with MCs even in the thoracic spine.
Modic changes (MCs) are signal intensity changes of vertebral bone marrow adjacent to the endplates of degenerated intervertebral discs on magnetic resonance images (MRI). Modic et al.13) first classified MCs into three types based on MRI findings and histological correlations. Modic type I changes (low SI on T1W images and high SI on T2W images) are associated with vascularized granulation tissue within subchondral bone, and indicate an ongoing active degenerative process. Modic type II changes (high SI on T1W and T2W images) reflect fatty replacement of adjacent marrow, and type III changes (low SI on T1W and T2W images) are believed to be associated with subchondral bone sclerosis on plain radiographs. In recent years, MCs have been extensively used to identify the causes of non-specific low back pain (LBP)2,5,6,8), and in particular, Modic type I change has been reported to be associated with LBP11,17).
Although MCs are more common in the lumbar spine, they are also found in the cervical and thoracic spine4,10,15). However, the majority of studies have focused on MCs of the lumbar spine, and more recently, on MCs of the cervical spine, and little information is available on the prevalence of MCs in thoracic spine. In fact, only one study conducted on 40 patients has described MCs in the thoracic spine4). Therefore, the present study was undertaken to evaluate further the prevalence, types, and locations of MCs in the thoracic spine in a large number of subjects, and to investigate the relation between the distributions of MCs and disc herniations (DHs) in the thoracic spine.
We retrospectively reviewed the records of 542 consecutive patients who underwent MRI of the thoracic spine at our hospital from January 2007 to December 2011. The study inclusion criteria were an age greater than 20 years, the receipt of thoracic MRI for the evaluation of a non-specific upper back pain at one imaging center using the same MRI equipment (Magnetom Avanto 1.5 T; Siemens Medical Solutions, Erlangen, Germany). The exclusion criteria included the presence of a pathologic condition other than degenerative disc disease (spinal infection, tumor, recent fracture or dislocation, intervening surgery, congenital block vertebrae, and scoliosis with a curvature of >15°).
Of the 542 patients, 144 fulfilled the inclusion criteria. Ages ranged from 22 to 88 years (mean±SD, 53.3±14.66 years). Seventy-two patients were female (50%). Thoracic MR images were retrospectively and independently analyzed by two experienced radiologists about the presence or absence of MCs and DHs. Thoracic segments ranged from T1/2 to T12/L1. Therefore, 1728 thoracic segment in 144 patients were evaluated. The type of MC was determined using the Modic classification13). DHs included both disc protrusion and extrusion, but excluded bulging. The ages and sexes of patients with MCs, and the prevalence, types, and distributions of MCs were recorded. In addition, the prevalence and distributions of DHs was recorded and relations between locations of MCs and DHs were explored using the chi-square test (SPSS version 12.0 for Windows; SPSS Inc., Chicago, IL, USA). Statistical significance was accepted for p values <0.05.
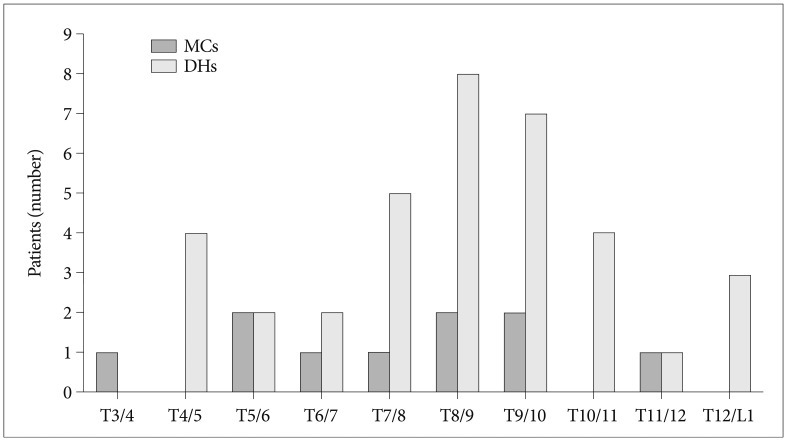
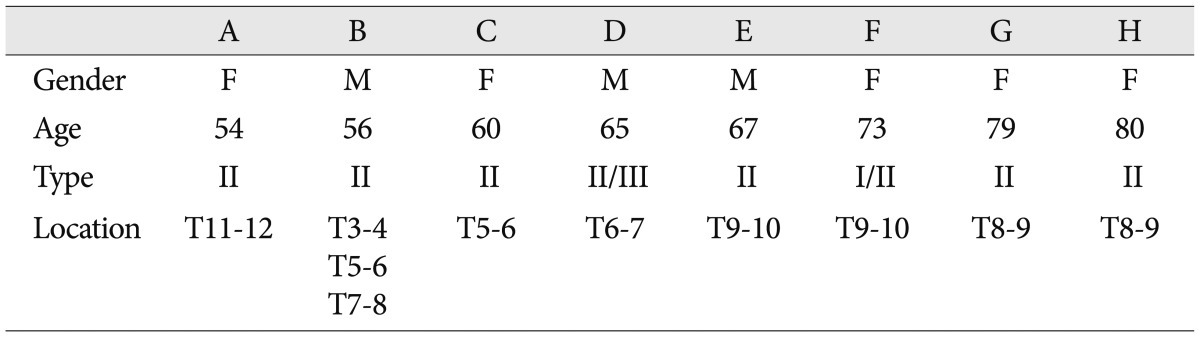
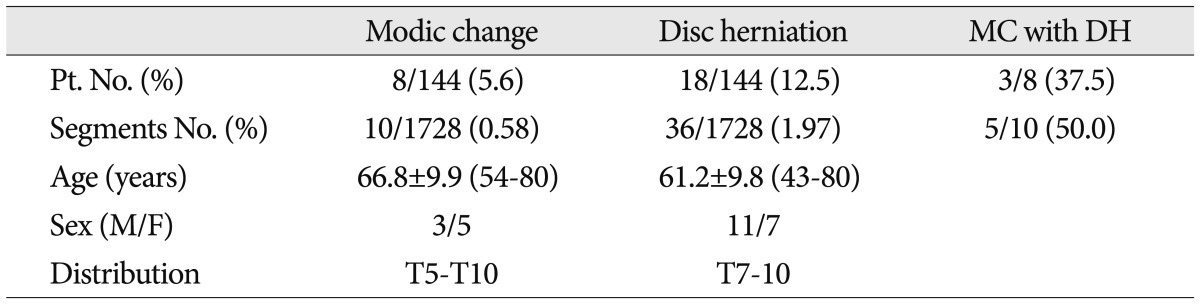
MC was observed in 8 of the 144 patients (5.6%) and in 10 of 1728 segments (0.58%). One patient had marrow changes in three segmental levels. The 8 patients included 3 men and 5 women of overall average age 66.8 years (range, 54-80 years). The most common MC detected was type II. Of the 8 patients with MC, 6 had type II (75.0%) and 2 had mixed modic lesions (type I/II or type II/III) (Fig. 1). No patient exhibited a typical type I or III marrow change (Table 1). Of the 1728 motion segments evaluated, 10 segments showed MC (0.58%), and all 10 were of type 2 (0.06%). MCs were located mainly at the mid-thoracic level (from T5/6 to T9/10) (Fig. 2).
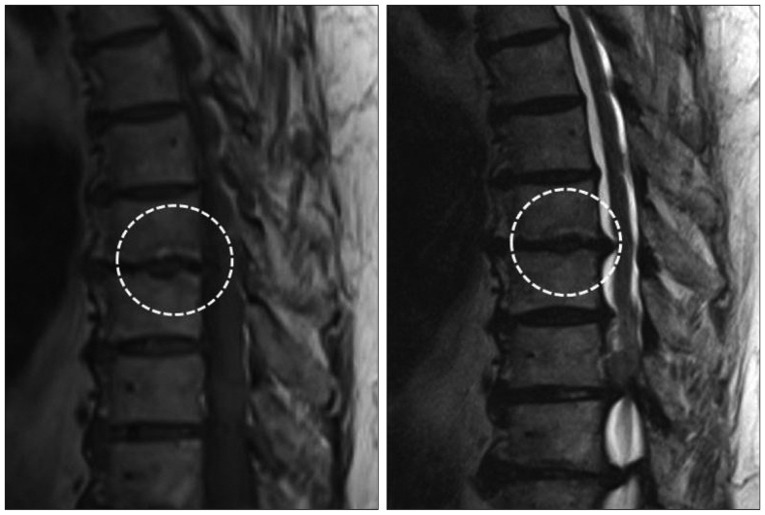
DHs were detected in 18 (12.5%) of the 144 patients and 36 (2.1%) of the 1728 segments. Of these 18 patients, 7 (38.9%) had a DH at more than one level, and 11 men and 7 women of overall average age 61.2 years (range, 43-80 years) were affected. The most commonly affected segments were T8/9 (n=8 DHs) and T9/10 (n=7 DHs). Of the 10 segments exhibiting MC, 5 had DH at the same level (50.0%) (Fig. 3, Table 2). Accordingly, DHs were found to be strongly associated with MCs (p= 0.000). The prevalence of patients with MC and DH at the same level was 3.5% (5 of the 144 patients).
The prevalence of MC in patients with a degenerative disc disease of the lumbar spine ranges has been reported to range from 19 to 59%13,17,19,20). Two recent studies on MC in cervical spine reported prevalence of 16% and 40.4%, respectively, which are similar to values for the lumbar spine10,15). In the present study, the overall prevalence of MCs in the thoracic spine was relatively low at 5.6% in our 144 patients. Girard et al.4) only described the MCs of the thoracic spine in total 40 patients and they also reported a low prevalence of 2.5%. Although the etiology of MCs is unknown, biomechanical factors are believed to be primarily responsible. In particular, biomechanical stress or instability could induce morphological changes, such as, microfractures or structural disorganization, in bone marrow adjacent to intervertebral discs9), which implies that the cervical and lumbar spines are more likely to be affected due to their wider ranges of motion. On the other hand, the rib cage decreases segmental motion in the thoracic spine and protects it from biomechanical stresses4). Therefore, the low prevalence of MC in thoracic spine observed in the present study could be due to a relative lack of mobility.
In the present study, type II MC was most common in the thoracic spine, and accounted for 75% of MCs. In addition, two mixed type MCs were observed, namely, types I/II and II/III. This result is quite different from that of the previous report, in which only one type I MC was observed4). The majority studies about MCs in lumbar spine have reported type II is most common, and that it may account for up to 80%1,7,13,16,20). In the cervical spine, Mann et al.10) also found that the most common type was type II. On the other hand, others have reported that type I is more common than type II in the lumbar and cervical spine7,15). However, few type I MCs remain stable and most convert to type II over several years8,11), which suggests that type I represents an active process. In contrast, type II MCs may not alter with time. Due to a lack of mobility of thoracic segments, MC progression is not common in the thoracic spine. Furthermore, the present study was performed on a larger population than the previous study, and thus, our finding that type II is the most common type in the thoracic spine is probably more accurate.
MCs most commonly occur at L4/5 and L5/S1 in lumbar spine8,13,20), whereas in the cervical spine, C5/6 and C6/7 are most affected10,15). These levels also represent the most mobile segments and commonly exhibit disc bulges or herniations. Girard et al.4) reported that the most commonly affected sites were T7-10 and T11-12, whereas we found that MCs were occurred mainly at mid-thoracic levels (T5-T10) and that did not distribute in any focal segment unlike cervical or lumbar spine. Although segmental motion of thoracic spine is less than that of other spinal regions, there is a slight difference of segmental motions according thoracic levels. Fujimori et al.3) is an in vivo 3-dimensional study reported that axial rotations of thoracic segments are significantly larger for middle segments (T6-T11) than upper or lower segments. This may be due to the stabilizing effect of the rib cage. Furthermore, scapulars help stabilize upper thoracic segments and at thoracolumbar junctions, axial rotation may also restricted by sagittalization of the facet joint14). Therefore, the segments found to exhibit MC in the thoracic spine appear to be concordant with segments with larger axial rotations. Consequently, like MCs in the cervical and lumbar spines, MCs in the thoracic spine may be associated with segment mobility.
MC is associated with degenerative disc diseases and commonly co-occurs in lumbar segments with DH1,7,13,20). Signal intensity changes (MCs) in vertebral body marrow adjacent to the endplates of degenerated disks are a common observation on MR images and appear to take three main forms12). On the other hands, herniated disks in the craniocaudal (vertical) direction through a break in the vertebral body endplate are reffered to as intravertebral herniations (Schmorl node). Most Schmorl node probably form after axial loading trauma, with preferential extrusion of nuclear material through the vertebral endplate rather than an intact and normal annulus fibrosis12). Degenerative marrow changes can occur surrounding the Schmorl node and expecially, type I MCs have been described surrounding the acute Schmorl node18).
In the present study, DH was detected in 18 patients (12.5%) and 34 segments (2.1%). Furthermore, the prevalence of DH in the thoracic spine was also found to be lower than in the cervical or lumbar spines, and DHs mainly occurred at T7-T11, which is concordant with greater segment mobility. Actually, DH accompanied MC in 50% of segments exhibiting MC. This finding suggests that DH is strongly associated with MC, even in the thoracic spine.
During recent years, MCs have been investigated as a cause of non-specific LBP2,5,6,8), and type I MC has been shown to be associated with LBP11,17). Toyone et al.17) reported that 73% of patients with type I MC had significant LBP, whereas only 11% of patients with type II MC had LBP. Mitra et al.11) concluded that LBP improves after type I progresses to type II, and Braithwaite et al.2) found that provocative discography increased LBP in patients with MC. In present study, we failed to find a correlation between MC and thoracic back pain because patients with nonspecific thoracic back pain were included, and no accurate diagnostic test, such as, provocative discography, was conducted. Furthermore, no patient in the present study exhibited typical type I MC, which is known to be strongly associated with LBP. Therefore, MC may not be clinically significant as a cause of thoracic back pain, considering the rarity of type I MC in thoracic spine.
In the present study, the prevalence of MC in the thoracic spine was low, and therefore, the statistical analysis was limited. Furthermore, MC in the thoracic spine may not be clinically significant. However, we believe that our study is meaningful, because it describes the prevalence of MC in the thoracic spine in a large number of subjects, and describes for the first time the distribution pattern of MCs and relations between MCs and DHs in thoracic spine and similarities between these relations and those between MCs and DHs in the cervical and lumbar spines.
In the present study, a low prevalence of MCs was observed in the thoracic spine with a type II predominance. The low prevalence of MC in the thoracic spine is suggested to be due to a relative lack of mobility. Nevertheless, despite the comparative rigidities of thoracic segments, MCs in thoracic spine appear to affect the more mobile mid-thoracic segments, which concur with that observed in the cervical and lumbar spines. Furthermore, DHs were found to be strongly associated with MC even in the thoracic spine.
References
1. Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007; 16:977–982. PMID: 17334791.

2. Braithwaite I, White J, Saifuddin A, Renton P, Taylor BA. Vertebral end-plate (Modic) changes on lumbar spine MRI : correlation with pain reproduction at lumbar discography. Eur Spine J. 1998; 7:363–368. PMID: 9840468.

3. Fujimori T, Iwasaki M, Nagamoto Y, Ishii T, Kashii M, Murase T, et al. Kinematics of the thoracic spine in trunk rotation : in vivo 3-dimensional analysis. Spine (Phila Pa 1976). 2012; 37:E1318–E1328. PMID: 22772578.
4. Girard CJ, Schweitzer ME, Morrison WB, Parellada JA, Carrino JA. Thoracic spine disc-related abnormalities : longitudinal MR imaging as sessment. Skeletal Radiol. 2004; 33:216–222. PMID: 14991248.
5. Jensen RK, Leboeuf-Yde C, Wedderkopp N, Sorensen JS, Jensen TS, Manniche C. Is the development of Modic changes associated with clinical symptoms? A 14-month cohort study with MRI. Eur Spine J. 2012; 21:2271–2279. PMID: 22526703.

6. Jensen TS, Karppinen J, Sorensen JS, Niinimäki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change) : a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008; 17:1407–1422. PMID: 18787845.

7. Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen JS, Bendix T. Magnetic resonance imaging and low back pain in adults : a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976). 2005; 30:1173–1180. PMID: 15897832.

8. Luoma K, Vehmas T, Grönblad M, Kerttula L, Kääpä E. MRI follow-up of subchondral signal abnormalities in a selected group of chronic low back pain patients. Eur Spine J. 2008; 17:1300–1308. PMID: 18648860.

9. Malinin T, Brown MD. Changes in vertebral bodies adjacent to acutely narrowed intervertebral discs : observations in baboons. Spine (Phila Pa 1976). 2007; 32:E603–E607. PMID: 17906561.
10. Mann E, Peterson CK, Hodler J. Degenerative marrow (modic) changes on cervical spine magnetic resonance imaging scans : prevalence, inter-and intra-examiner reliability and link to disc herniation. Spine (Phila Pa 1976). 2011; 36:1081–1085. PMID: 21224758.

11. Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol. 2004; 14:1574–1581. PMID: 15060839.

12. Modic MT, Ross JS. Lumbar degenerative disk disease. Radiology. 2007; 245:43–61. PMID: 17885180.

13. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease : assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988; 166(1 Pt 1):193–199. PMID: 3336678.

14. Panjabi MM, Oxland T, Takata K, Goel V, Duranceau J, Krag M. Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1993; 18:1298–1310. PMID: 8211362.

15. Peterson CK, Humphreys BK, Pringle TC. Prevalence of modic degenerative marrow changes in the cervical spine. J Manipulative Physiol Ther. 2007; 30:5–10. PMID: 17224349.

16. Ross JS, Perez-Reyes N, Masaryk TJ, Bohlman H, Modic MT. Thoracic disk herniation : MR imaging. Radiology. 1987; 165:511–515. PMID: 3659375.
17. Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J Bone Joint Surg Br. 1994; 76:757–764. PMID: 8083266.

18. Wagner AL, Murtagh FR, Arrington JA, Stallworth D. Relationship of Schmorl’s nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am J Neuroradiol. 2000; 21:276–281. PMID: 10696008.
19. Wang Y, Videman T, Battié MC. Modic changes : prevalence, distribution patterns, and association with age in white men. Spine J. 2012; 12:411–416. PMID: 22515998.

20. Wu HL, Ding WY, Shen Y, Zhang YZ, Guo JK, Sun YP, et al. Prevalence of vertebral endplate modic changes in degenerative lumbar scoliosis and its associated factors analysis. Spine (Phila Pa 1976). 2012; 37:1958–1964. PMID: 22565387.

Fig. 1
Thoracic MRI of a 73-year-old man with mixed type Modic change (type I/II) at T9-10. A : Sagittal T1-weighted image revealing high signal intensity of the lower endplate of T9 and low signal intensity of the upper endplate of T10. B : Sagittal T2-weighted image showing high signal intensity of both endplates at T9-10.
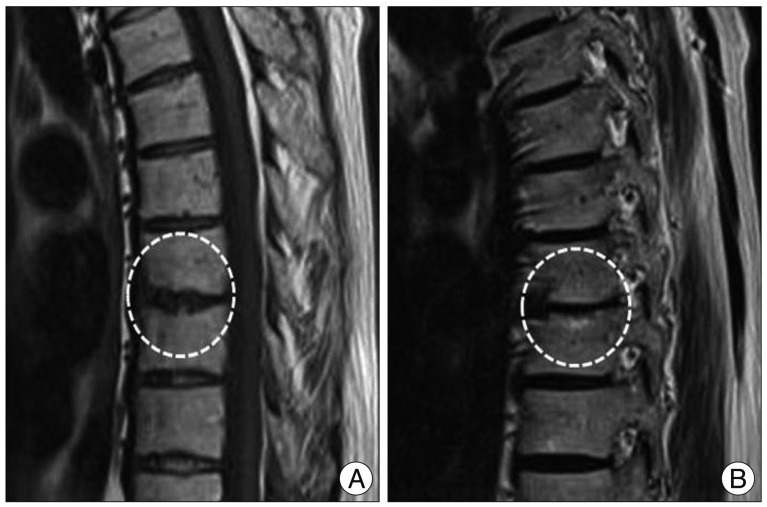
Fig. 2
Distribution of modic changes and disc herniations in the thoracic spine. MC : Modic change, DH : disc herniation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download