Abstract
Objective
Cerebral vasospasm is a common and potentially devastating complication of aneurysmal subarachnoid hemorrhage (aSAH). Inflammatory processes seem to play a major role in the pathogenesis of vasospasm. C-reactive protein (CRP) constitutes a highly sensitive inflammatory marker. Elevation of serum CRP levels has been demonstrated in patients with aSAH. The purpose of the current study was to evaluate the possible relationship between CRP levels in the serum and transcranial Doppler (TCD) and the development of vasospasm in patients with aSAH.
Methods
A total of 61 adult patients in whom aSAH was diagnosed were included in the study from November 2008 to May 2011. The patients' demographics, Hunt and Hess grade, Fisher grade, CT scans, digital subtraction angiography studies, and daily neurological examinations were recorded. Serial serum CRP measurements were obtained on days 1, 3, 5, 7, 9, 11 and 13 and TCD was measured on days 3, 5, 7, 9, 11 and 13. All patients underwent either surgical or endovascular treatment within 24 hours of their hemorrhagic attacks.
Results
Serum CRP levels peaked on the 3rd postoperative day. There were significant differences between the vasospasm group and the non-vasospasm group on the 1st, 3rd and 5th day. There were significant differences between the vasospasm group and the non-vasospasm group on the 3rd day in the mean middle cerebral artery velocities on TCD.
Rupture of an intracranial aneurysm carries a high risk of death or disability. A previous international study reported that of the patients who survived the initial ictus, 33% were rendered severely disabled, vegetative, or dead after aneurysmal subarachnoid hemorrhage (aSAH)19). Despite recent advances in treatment modalities, such as an endovascular coil embolization, outcomes for patients with a ruptured aneurysm remains unchanged2). Approximately 50% of patients suffering from an aSAH will die, 15% of them will become severely disabled, and only 20-35% will return to normal life and activities1,8,11,28).
Cerebral vasospasm remains the most troublesome complication of aSAH. It is associated with high morbidity and mortality, even after successful treatment of the ruptured aneurysm. The occurrence of cerebral vasospasm varies significantly. It has been demonstrated to be as high as 70% based on angiography, and in 20-30% of the patients, vasospasm is responsible for the development of a delayed ischemic neurological deficit11,18). Several theories have been proposed in an attempt to explain the underlying pathophysiological mechanisms behind cerebral vasospasm7,12,13,21,25,30). A relatively recent theory postulates that an inflammatory mechanism is implicated in the development of coronary artery vasospasm8). Considerable indirect evidence has been gathered that suggests that vasospasm may be the result of an inflammatory process taking place in the arterial wall that is initiated by the surrounding clot14,18,22,31). Morphological changes compatible with an inflammatory process15,22,33), leukocytes located in the blood vessel wall6) and adherent to the endothelial surface, the multiple complex origins of vasospasm29), experimental responses to non-steroidal anti-inflammatory drugs32) and steroids4), and studies of the inflammatory process5,24) all support this hypothesis.
C-reactive protein (CRP) is an acute phase sensitive, non-specific inflammatory marker and initiating factor in inflammation and infection. Previous large scale studies, including meta-analyses have found modestly elevated CRP levels are a risk factor for coronary heart disease, as well as for both vascular and non-vascular mortality, independent of confounding factors3,9). It is assumed that CRP plays an active role in the atherosclerotic process. The protein induces expression of different adhesion molecules on endothelial cells and is able to activate complement within the vessel wall. Furthermore, the recent discovery of local production of CRP and complement proteins within the atherosclerotic plaque suggests an active role in the pathogenesis of vascular degenerative processes27).
In current clinical practice, transcranial Doppler (TCD) examination and electroencephalography monitoring is recommended in patients at high risk for vasospasm and impaired consciousness. Digital subtraction angiography is the gold standard for the diagnosis of cerebral vasospasm and is recommended when there is no response to medical treatment and the patient is a candidate for endovascular therapy26).
A TCD study is a noninvasive and early diagnostic method for vasospasm and is performed primarily in aSAH, however, it has some disadvantages. TCD is often normal during the period of highest risk for vasospasm or there is a discrepancy between clinical and sonographic findings.
This study aimed to elucidate whether CRP deserves to be used for the early diagnosis of vasospasm in aSAH.
We prospectively collected the medical records of all patients who were examined between November 2008 and March 2011 and who had been hospitalized for acute aSAH in our department. We excluded all patients who had a previous SAH, an acute infectious disease, and those with a previous operation within 10 days. All patients underwent surgical treatment or endovascular treatment within 24 hours of the onset of the SAH.
Among the 61 patients, the male to female ratio was 1 : 3 and the mean age of the patients was 52.5 years (range, 26-81). The records of these 61 patients included demographic data, Hunt & Hess grade, and Fisher grade. Patients were classified according to the location of the lesion, postoperative infection, and surgical methods used (Table 1).
We obtained postoperative blood samples from the patients for CRP levels on the 1st, 3rd, 5th, 7th, 9th, 11th and 13th days. We also performed TCD on the 3rd, 5th, 7th, 9th, 11th, and 13th postoperative days and neurological examinations daily after admission. Cerebral vasospasm was diagnosed when a new neurologic deficit developed or when the mean TCD velocity was higher than 120 cm/sec. We excluded other conditions that can make patients' neurologic state deteriorate such as hydrocephalus, seizure, electrolyte imbalance etc.
We redistributed the Hunt and Hess grades and Fisher grades for the two groups. The Hunt and Hess grades were divided into 1, 2 and 3, 4, 5, while the Fisher grades were 1, 2, 3, and 4. After surgery, all patients were screened for evidence of infections such as cystitis, pneumonia, sinusitis, or other inflammation. The patient was classified as having an infection when either a microbiologic specimen indicated an infection or when antibiotic treatment was initiated.
1) Correlations between the CRP levels measured on the 1st, 3rd, 5th, 7th, 9th, 11th, and 13th postoperative days and vasospasm were analyzed.
2) Correlations between the TCD measured on the 3rd, 5th, 7th, 9th, 11th and 13th postoperative days and vasospasm were analyzed.
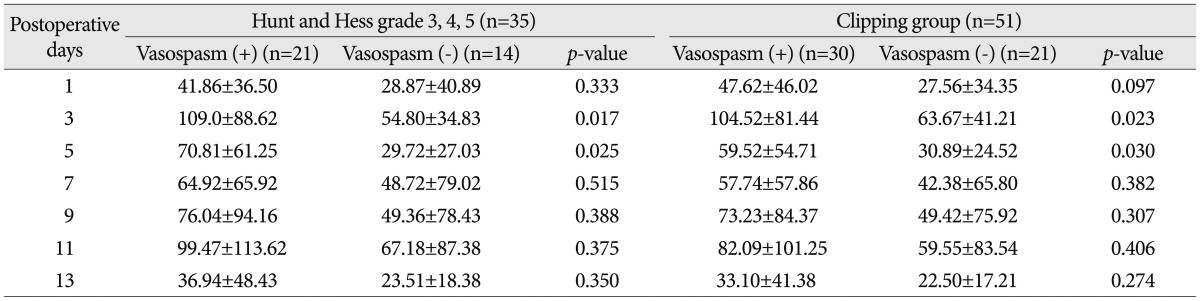
3) The Hunt and Hess grades were divided into 1, 2 and 3, 4, 5. Using this division, we compared the CRP patterns of the two groups. The CRP patterns were compared according to the vasospasm occurrence within Hunt and Hess grade 3, 4, 5.
4) We compared the CRP pattern of the two groups based on a division of the Fisher grades into 1, 2, 3 and grade 4.
5) Patients were divided into an aneurysmal clipping group and a coiling group and the CRP patterns of the two groups were compared.
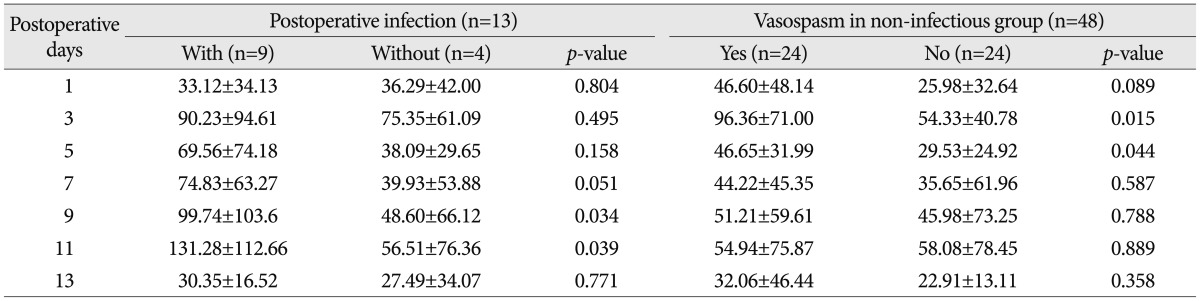
6) We analyzed CRP levels of the group with infection during treatment. To exclude the effects of an infection, we analyzed the correlation between CRP levels and the occurrence of vasospasm in the group without infection.
7) Finally we calculated a predictability cutoff value for the prediction of vasospasm based on CRP and TCD.
All patients were evaluated for a baseline value. However, some subjects had missing data for the outcome variables on the second postoperative day. For subjects with missing outcomes on the 1st day after surgery, missing data were replaced with the mean value of each group at that time. After the 1st day, missing data were completed using an last observation carrying forward analysis.
For intergroup comparisons, the distribution of the data was first evaluated for normality using the Shapiro-Wilk test. Normally distributed data were compared using Student's t-test. Non-normally distributed data were analyzed using the Mann-Whitney U test. Descriptive variables were subjected to chi-square analysis or Fisher's exact test, as appropriate.
Data in the manuscript are presented as the mean±standard deviation and data in the figures are reported as the mean±standard error. p<0.05 was considered statistically significant. Statistical analysis was conducted using SPSS version 18.0 (IBM Corp., Armonk, NY, USA). We used Receiver Operating Characteristic (ROC) curves for predicting cutoff values. ROC curve is a graphical representation of the trade of between the sensitivity and 1-specificity rates for every possible cut-off. The closer the ROC plot is to the upper left corner, the greater the overall accuracy of the test. Therefore the accuracy of a test is measured by the area under the ROC curve (AUC). The asymptotic Sig. (P) less than 0.05 can be a useful test method. Testing both the lower and upper bounds of the asymptotic 95% confidence interval for whether they are more than 0.05 can be useful. Researchers select in appropriate point of their choice and then decide on a cut-off value using the statistical data.
Among the 61 patients with SAH, 33 patients showed clinical or radiologic vasospasm. CRP levels were elevated significantly on the 1st, 3rd, and 5th postoperative days in the vasospasm group (Table 2, Fig. 1), and the mean TCD values for the middle cerebral artery were significantly higher on the 3rd, 5th, 9th, 11th, and 13th postoperative days in the vasospasm group (Table 3, Fig. 2).
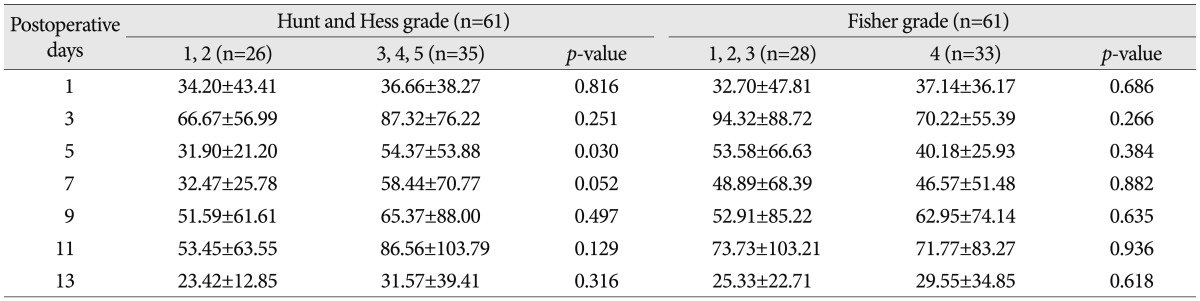
When classifying patients according to Hunt & Hess grades, CRP levels were elevated significantly on the 5th postoperative day in the Hunt & Hess grades 3, 4 and 5 group (Table 4). In the Hunt & Hess grades 3, 4 and 5 group, when the correlations between the CRP measured on postoperative days of 3rd, 5th, 7th, 9th, 11th, and 13th and vasospasm were assessed, CRP levels were significantly different between vasospasm and non-vasospasm group, on postoperative days 3 and 5 (Table 5). There was no statistically significant difference in CRP levels between the groups with Fisher grades 1, 2, and 3 and the group with grade 4 (Table 4). In patients with aneurysmal clipping, the CRP levels were significantly higher in the vasospasm group on postoperative days 3 and 5 (Table 5). However, CRP elevation can also occur by infection and the bias caused by this possibility should be removed. For this, patients were divided into two groups-one is those who experienced infection during treatment after surgery and the other is who did not have such an experience. Each group's CRP and vasospasm was compared. As a result, the CRP levels were significantly higher in the group with infection on postoperative days 9 and 11. When compared in patients without infection, the CRP levels were significantly higher in the group with vasospasm on the 3rd and 5th postoperative days (Table 6, Fig. 3).
Based on these outcomes, we estimated the predictability cutoff value for CRP levels on the first day after the surgery in patients with a higher chance of vasospasm. We also estimated the predictability cutoff value of the mean TCD velocity on the 3rd postoperative day (Fig. 4). The AUC to predict vasospasm were 0.700 for CRP levels on the 1st postoperative day, so accuracy was reasonable. The asymptotic Sig. (P) was 0.008, so the testing methods were useful. Both the lower and upper bounds of the asymptotic 95% confidence interval were 0.567 and 0.832, so the testing methods were useful. We determined the appropriate CRP values measured on the 1st postoperative day that can predict vasospasm. When CRP values were 26.5 mg/L, the sensitivity and specificity were 60.6% and 78.6%.
Similarly, the AUC to predict vasospasm were 0.822 for the mean TCD level on the 3rd postoperative day, so accuracy was reasonable. Asymptotic Sig. (P) was 0.000, so testing methods were useful. And both the lower and upper bounds of the asymptotic 95% confidence interval were 0.714 and 0.930, so the testing methods were useful. We determined the appropriate mean TCD level measured on the 3rd postoperative day that can predict vasospasm. When the mean TCD levels were 75.5 cm/sec, the sensitivity and specificity were 78.8% and 75.0%.
The role of inflammation in the development and maintenance of cerebral vasospasm has been previously demonstrated10). Several large scale studies have shown that elevated CRP levels may reflect an increased risk for myocardial or cerebral infarction, as well as for both vascular and non-vascular mortality3,9). CRP levels correlate with severity and outcomes of several diseases3,9,23). CRP is mainly synthesized in the liver after induction by cytokines, particularly by interleukin-6, and it activates the complement system contributing to natural immunity23). An elevated CRP level is the epiphenomenon of aSAH and has been estimated to be a marker for the extent of atherosclerosis or inflammatory activity and for vulnerability of atherosclerotic plaques. CRP could also directly contribute to the development of ischemic cardiovascular or cerebrovascular disease. This may suggest that inflammatory mechanisms could contribute to secondary neuronal injury after cerebral ischemia17). In one study CRP levels in both plasma and cerebrospinal fluid (levels higher than in plasma) was elevated to peak levels before angiographic vasospasm10). Similarly, early phase CRP levels after SAH have predicted delayed ischemia or angiographic vasospasm in two studies10,20).
In our study, CRP levels were significantly higher on the 1st, 3rd, and 5th postoperative days in the vasospasm group. This result reflects that the inflammatory processes contribute to cerebral vasospasm. Mean TCD values were significantly higher in the vasospasm group all days after surgery, but the mean TCD values peaked on the 9th postoperative day. The mean TCD values over 120.0 cm/sec, which could be diagnosed as vasospasm, occurred on the 9th postoperative day as well. Compared to the CRP levels, the TCD values were elevated a little later in the development of vasospasm.
An increase in the postoperative CRP was associated with the time profile of the development of symptomatic vasospasm, and a CRP postoperative 1 and 2 days cutoff point of 25 mg/L seemed to have a moderate sensitivity and specificity in predicting symptomatic vasospasm16). CRP levels in our study were significantly higher in the vasospasm group on postoperative days 3 and 5 in the Hunt & Hess grades 3, 4 and 5 group. According to CT findings, there was no significant difference in CRP levels between the groups with Fisher grade 1, 2, and 3 and grade 4. This means that CRP levels do not reflect the degree of inflammatory reaction according to the amount of hemorrhage.
Considering the treatment methods of coil embolization and surgical clipping, the surgical procedure might elevate CRP levels. In order to distinguish between a post-surgical and a vasospastic CRP elevation, a correlation between the levels and vasospasm was assessed in only patients with aneurysm clipped. CRP levels were found to be significantly higher in the vasospasm group on postoperative days 3 and 5. Regardless of the surgical methods, we postulate that the CRP level was increased due to vasospasm.
CRP levels were higher in the group with infection on the 9th and 11th postoperative days. In patients without infection, CRP levels were higher in the group with vasospasm on the 3rd and 5th postoperative days. This result could be interpreted as a period needed for the elevation of CRP levels in patients with infection after surgery. It appears that the infectious inflammatory reaction develops later than vasospasm. As a result, an elevated CRP level on the 3rd and 5th postoperative days seemed to be due more to vasospasm than to infection.
Based on the above results, we estimated a predictability cutoff value for the prediction of vasospasm by CRP levels on the 1st postoperative day. A cut-off point for CRP levels for predicting vasospasm on the 1st postoperative day was 26.5 mg/L with a sensitivity of 60.6% and a specificity of 78.6%. A cut-off point for the mean TCD values for predicting the vasospasm on the 3rd postoperative day were 75.5 cm/sec with a sensitivity of 78.8% and a specificity of 75%. These are moderate predictability for vasospasm, however combining the CRP and TCD value deserves to be used for the early diagnosis of vasospasm in aSAH.
References
1. Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage : a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009; 40:994–1025. PMID: 19164800.

2. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006; 355:928–939. PMID: 16943405.

3. Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease : a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009; 151:483–495. PMID: 19805771.

4. Chyatte D, Rusch N, Sundt TM Jr. Prevention of chronic experimental cerebral vasospasm with ibuprofen and high-dose methylprednisolone. J Neurosurg. 1983; 59:925–932. PMID: 6631514.

5. Cotran RS. The endothelium and inflammation : new insights. Monogr Pathol. 1982; 18–37. PMID: 6181396.
6. Crompton MR. The pathogenesis of cereral infarction following the rupture of cereral berry aneurysms. Brain. 1964; 87:491–510. PMID: 14215175.

7. Dietrich HH, Dacey RG Jr. Molecular keys to the problems of cerebral vasospasm. Neurosurgery. 2000; 46:517–530. PMID: 10719847.

8. Dumont AS, Dumont RJ, Chow MM, Lin CL, Calisaneller T, Ley KF, et al. Cerebral vasospasm after subarachnoid hemorrhage : putative role of inflammation. Neurosurgery. 2003; 53:123–133. discussion 133-135. PMID: 12823881.
9. Elkind MS, Luna JM, Moon YP, Liu KM, Spitalnik SL, Paik MC, et al. High-sensitivity C-reactive protein predicts mortality but not stroke : the Northern Manhattan Study. Neurology. 2009; 73:1300–1307. PMID: 19841382.

10. Fountas KN, Tasiou A, Kapsalaki EZ, Paterakis KN, Grigorian AA, Lee GP, et al. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus. 2009; 26:E22. PMID: 19409001.
11. Haley EC Jr, Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992; 23:205–214. PMID: 1561649.

12. Hansen-Schwartz J. Cerebral vasospasm : a consideration of the various cellular mechanisms involved in the pathophysiology. Neurocrit Care. 2004; 1:235–246. PMID: 16174921.

13. Harrod CG, Bendok BR, Batjer HH. Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage : a review. Neurosurgery. 2005; 56:633–654. discussion 633-654. PMID: 15792502.

14. Hoshi T, Shimizu T, Kito K, Yamasaki N, Takahashi K, Takahashi M, et al. [Immunological study of late cerebral vasospasm in subarachnoid hemorrhage. Detection of immunoglobulins, C3, and fibrinogen in cerebral arterial walls by immunofluorescence method]. Neurol Med Chir (Tokyo). 1984; 24:647–654. PMID: 6083487.

15. Hughes JT, Schianchi PM. Cerebral artery spasm. A histological study at necropsy of the blood vessels in cases of subarachnoid hemorrhage. J Neurosurg. 1978; 48:515–525. PMID: 632876.
16. Jeon YT, Lee JH, Lee H, Lee HK, Hwang JW, Lim YJ, et al. The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2012; 24:317–324. PMID: 22732721.

17. Juvela S, Kuhmonen J, Siironen J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 2012; 154:397–404. PMID: 22134501.

18. Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985; 16:562–572. PMID: 3895589.

19. Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1 : Overall management results. J Neurosurg. 1990; 73:18–36. PMID: 2191090.
20. Kubo Y, Ogasawara K, Kakino S, Kashimura H, Tomitsuka N, Sugawara A, et al. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008; 69:592–596. discussion 596. PMID: 18486699.

21. Lin CL, Jeng AY, Howng SL, Kwan AL. Endothelin and subarachnoid hemorrhage-induced cerebral vasospasm : pathogenesis and treatment. Curr Med Chem. 2004; 11:1779–1791. PMID: 15279581.

22. Mizukami M, Kawase T, Tazawa T, Nagata K, Yunoki K, Yoshida Y. Hypothesis and clinical evidence for the mechanism of chronic cerebral vasospasm after subarachnoid hemorrhage. Cerebral Arterial Spasm. Baltimore: Williams & Wilkins;1980. p. 97–112.
23. Nordestgaard BG, Zacho J. Lipids, atherosclerosis and CVD risk : is CRP an innocent bystander? Nutr Metab Cardiovasc Dis. 2009; 19:521–524. PMID: 19695857.
24. Pinckard RN. The "new" chemical mediators of inflammation. Monogr Pathol. 1982; 38–53. PMID: 6126808.
25. Pluta RM. Delayed cerebral vasospasm and nitric oxide : review, new hypothesis, and proposed treatment. Pharmacol Ther. 2005; 105:23–56. PMID: 15626454.

26. Rodríguez García PL, Rodríguez Pupo LR, Rodríguez García D. [Diagnosis of delayed cerebral ischaemia and cerebral vasospasm in subarachnoid haemorrhage]. Neurologia. 2010; 25:322–330. PMID: 20643043.

27. Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A. Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2006; 18:68–72. PMID: 16369143.

29. Towart R. The pathophysiology of cerebral vasospasm, and pharmacological approaches to its management. Acta Neurochir (Wien). 1982; 63:253–258. PMID: 7102416.

30. Umemoto S, Suzuki N, Fujii K, Fujii A, Fujii T, Iwami T, et al. Eosinophil counts and plasma fibrinogen in patients with vasospastic angina pectoris. Am J Cardiol. 2000; 85:715–719. PMID: 12000045.

31. Weir B, Okwuasaba F, Cook D, Krueger C. Pharmacology of vasospasm-Effects of various agents including blood on isolated cerebral arteries. Cerebral arterial spasm. Baltimore: Williams and Wilkins;1980. p. 237–243.
32. White RP, Robertson JT. Comparison of piroxicam, meclofenamate, ibuprofen, aspirin, and prostacyclin efficacy in a chronic model of cerebral vasospasm. Neurosurgery. 1983; 12:40–46. PMID: 6338410.

33. Yamashima T, Yamamoto S. Cerebral arterial pathology in experimental subarachnoid hemorrhage. J Neurosurg. 1983; 58:843–850. PMID: 6854377.

Fig. 1
Schematic representation of C-reactive protein (CRP, mg/L) levels in serum with and without vasospasm.
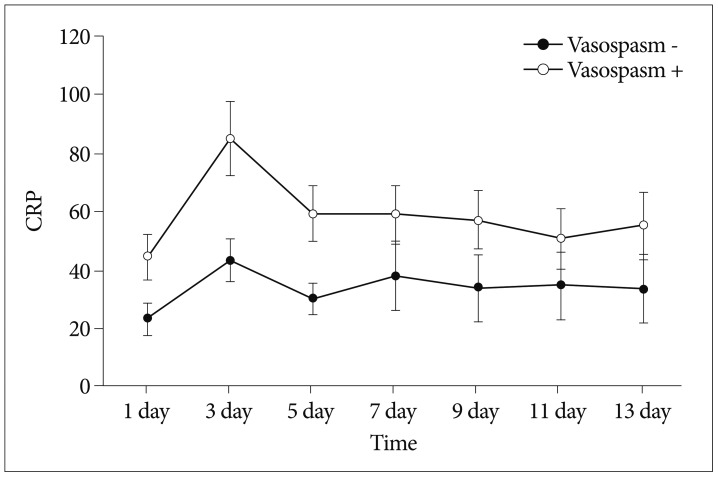
Fig. 2
Schematic representation of mean transcranial Doppler (TCD, cm/sec) levels with and without vasospasm.
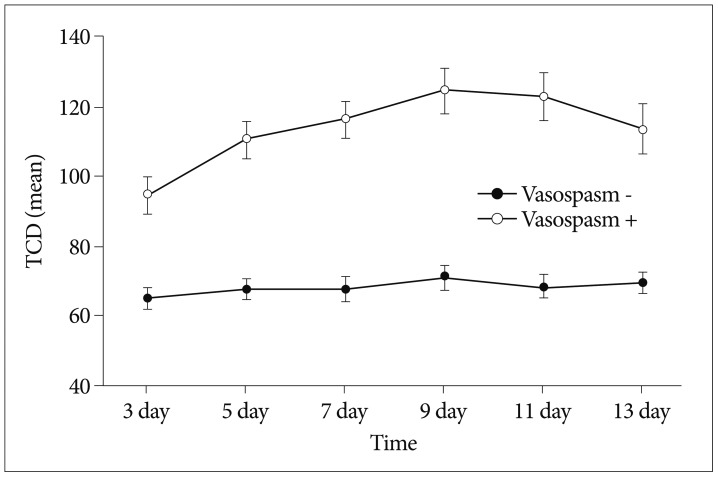
Fig. 3
Schematic representation of C-reactive protein (CRP, mg/L) levels according to the development of vasospasm in the postoperative infectious group (A) and in the non-infectious group (B). A : CRP levels are significantly higher in the group with infection on postoperative days 9 and 11. B : CRP levels are significantly higher in the group with vasospasm on the 3rd and 5th postoperative days.
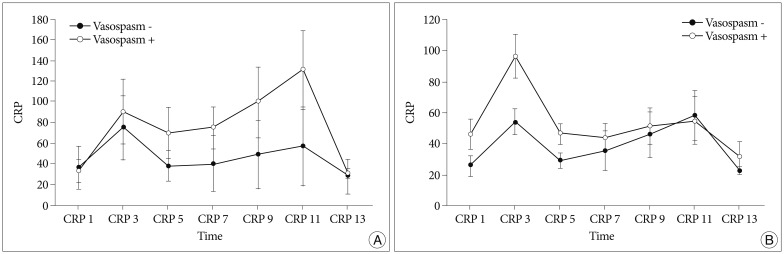
Fig. 4
Receiver Operating Characteristic (ROC) curve of C-reactive protein (CRP) levels in serum on the 1st postoperative day (A) and mean transcranial Doppler (TCD) on the 3rd postoperative day (B). A : The area under the receiver-operating curves to predict vasospasm is 0.700 for CRP levels on the 1st postoperative day. B : The area under the receiver-operating curves to predict vasospasm is 0.822 for the mean TCD level on the 3rd postoperative day. AUC : area under the ROC curve.
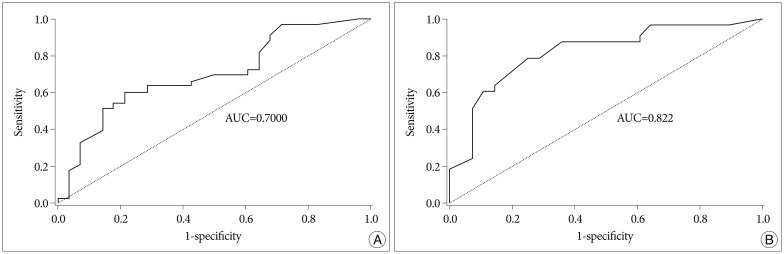
Table 2
Serial C-reactive protein levels (mg/L, mean±standard error) in the serum of patients with and without vasospasm
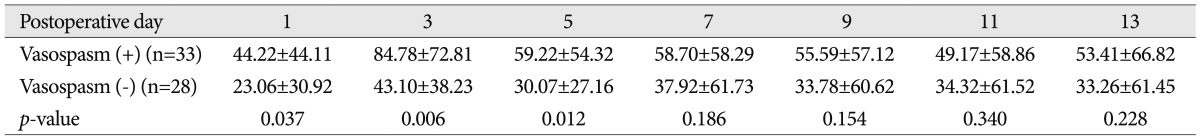
Table 3
Mean transcranial Doppler values measured in the middle cerebral artery (cm/sec, mean±standard error) in patients with and without vasospasm
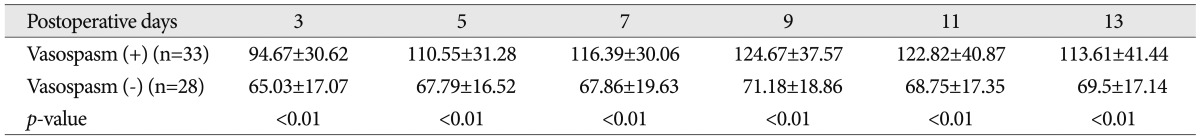
Table 4
Serial C-reactive protein levels (mg/L, mean±standard error) according to Hunt and Hess grade and Fisher grade




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download