Abstract
Microvascular decompression is a very effective and relatively safe surgical modality in the treatment of hemifacial spasm. But rare debilitating complications have been reported such as cranial nerve dysfunctions. We have experienced a very rare case of unilateral soft palate palsy without the involvement of vocal cord following microvascular decompression. A 33-year-old female presented to our out-patient clinic with a history of left hemifacial spasm for 5 years. On postoperative 5th day, patient started to exhibit hoarsness with swallowing difficulty. Symptoms persisted despite rehabilitation. Various laboratory work up with magnetic resonance image showed no abnormal lesions. Two years after surgery patient showed complete recovery of unitaleral soft palate palsy. Various etiologies of unilateral soft palate palsy are reviewed as the treatment and prognosis differs greatly on the cause. Although rare, it is important to keep in mind that such complication could occur after microvascular decompression.
Microvascular decompression (MVD) has been known to be an effective surgical modality as the treatment of various hyperactive neurovascular compression syndromes. Its high success rate (over 90% long term cure rate) in the treatment of hemifacial spasm (HFS) has made it the treatment of choice5,10). Although MVD is known to be a relatively safe procedure, it is rarely reported in the literature to accompany severe disabling complications. Common complications of MVD for HFS are also brainstem or cerebellar infarct, hematomas, cerebrospinal fluid leak and cranial nerve palsy, commonly involving 7th and 8th nerve with rare lower cranial nerve palsies are reported1,5,11). We have experienced unilateral soft palate palsy without the involvement of vocal fold after MVD for HFS. So far, to our knowledge, such complication is very rare, following MVD. Here, we report a complication of unilateral soft palate palsy after MVD.
A 33-year-old female was presented to our out-patient clinic with a history of left hemifacial spasm for 5 years. Her past medical history was unremarkable. The patient underwent microvascular decompression in 2010 at our medical center (Fig. 1).
Intra-operatively, bilateral facial electromyography and brain stem auditory evoked potential was monitored. After the dura incision, gentle retraction of the cerebellum was applied using Greenburg retractor and we have exposed the lower cranial nerves. Dissection of the arachnoid was done from the rostral border of the lower cranial nerves. The arachnoid band was very tough and difficult to dissect, but no obvious injury occurred to the lower cranial nerve during the dissection. The left anterior inferior cerebellar artery was the offending vessel compressing the facial nerve from the caudal side and decompression was successfully completed without any adverse events and with no change in the intraoperative monitoring of brainstem auditory evoked potential. Patient recovered well from anesthesia with reduced facial spasm, compared to the pre-operative state. Postoperatively, neurologic exam revealed no abnormalities and also patient's swallowing and vocalization function was intact. Immediate postoperative brain CT scan performed on postoperative day showed no sign of hemorrhage.
On postoperative 5th day, the patient started to exhibit hoarsness with swallowing difficulty. Barium swallowing test was done to evaluate dysphagia, and minimal nasal regurgitation was demonstrated due to unilateral soft palate palsy. On endoscopic exam, performed by an otolaryngologist, the vocal cord was intact in both sides with unilateral left soft palate palsy, which suggested tensor and/or levator veli palatini muscle weakness (Fig. 2). Under the impression of possible lower cranial nerve palsy caused by postoperative edema of the nerve, steroid (prednisolone) was prescribed and we decided to observe the symptoms. Symptoms persisted with slight improvement with steroid therapy but repeatedly aggravated with steroid tapering. She received speech rehabilitation therapy for persistent difficulty of phonation and swallowing.
On postoperative 19th day, patient complained of difficulty breathing during sleep. Polysomnography was done to evaluate sleep apnea. Mild obstructive sleep apnea syndrome was observed secondary due to soft palate palsy. We encouraged her to sleep on her side; then her symptom improved.
Despite persistent speech rehabilitation, symptoms still persisted. We performed cranial nerve MRI to evaluate for the possibility of any structural abnormalities, such as delayed hematoma or possible infection. However, MRI showed no abnormal structural lesions or abnormal enhancement, which may suggest infection (Fig. 3). The patient continued to receive speech rehabilitation with subjective improvement of swallowing and speech.
On postoperative 25th day, the patient developed grade II facial palsy by House-Brackmann grade on the left side. Patient's facial palsy improved with administration of acyclovir for 5 days, but hoarseness with swallowing difficulty showed little improvement.
The patient received prolonged speech and swallowing rehabilitation therapy, and follow up examination was done before discharge showed improvement of nasalization, but persistent hypernasality. Patient was discharged with recommendation for prolonged rehabilitation.
Patient visited our out-patient for a follow up in postoperative 2 years. Endoscopic examination revealed normalized soft palate palsy and no abnormality in the vocal cord mobility (Fig. 4). However, patient's subjective difficulty of speech still persisted and we decided to observe her symptoms for any changes in the future.
Cranial nerve palsy has been reported as complication of MVD and can cause symptoms that markedly decrease the quality of life. Most commonly, the 7th and 8th cranial nerve are involved with rare lower cranial nerve involvement. Among the lower cranial nerves, glossopharyngeal nerve and vagus nerve are most commonly involved.
Glossopharyngeal nerve contains both motor and sensory branches that supply the tongue and stylopharyngeus muscle of the pharynx. Impairment of glossopharyngeal nerve would cause decreased sensation in the pharynx including taste in the posterior one third of the tongue with difficulty in swallowing and dysphonia2-4). Our patient developed swallowing difficulty with dysphonia, which suggests possible dysfunction of the 9th nerve.
Vagus nerve also contains both motor and sensory branches that innervate all striated muscles of the laryngopharynx, except the stylopharyngeus and tensor veli palatini. Patients with unilateral high vagal lesions would suffer from breathy dysphonia and aspiration because of impaired vocal cord movement. Due to the loss of sensation of the laryngopharynx, causing loss of gag reflex, patients might also suffer severe dysphagia related to the poor coordination of the oro- and hypo-pharyngeal phases of swallowing2-4). Unilateral soft palate palsy with dysphonia and dysphagia was observed in our patient, which suggests unilateral vagus nerve dysfunction, but the sparing of the vocal cord suggest the function of the vagus nerve is only partially impaired.
Although the functions and innervations of the 9th and 10th nerve are known, it is difficult to exactly locate which lower cranial nerve is causing the symptom by physical examination. Many symptoms are subtle and overlap with other diseases, making the diagnosis elusive2).
Common injury to the vagus nerve is accompanied by vocal fold palsy, but our patient only presented with soft palate palsy. In addition, partial injury of the vagus nerve with possible involvement of the glossopharyngeal nerve have never been reported as a complication of MVD in prior literature, thus making it difficult to identify the exact cause of such peculiar symptoms.
Several etiologies are reported in literature that can cause lower cranial nerve paralysis. Skull base tumors are the most common cause and trauma or surgery above the skull base and from the cerebellopontine angle can also be a cause. Rarely, infection and ischemic vascular insult are reported to cause unilateral vagus nerve palsy3,7,8,12).
It is important to note that in our patient, the hoarsness and dysphagia developed several days after the surgery. Generally, lower cranial palsy caused by trauma would occur immediately after the event, which implies the possibility of other factors, such as infection contributing in the delayed onset of lower cranial nerve palsy.
In the literature, some have reported herpes simplex virus (HSV) to be a possible cause of unilateral vagus nerve palsy12). In addition, varicella zoster virus is also reported to cause lower cranial nerve palsy6). Although we have failed to show such connection in our patient, history of delayed facial palsy also suggests the possibility of concurrent viral infection. Others have also suggested that delayed facial palsy after MVD in patients with HFS may be caused by viral infections. As HSV is widely known to cause Bell's palsy, it could likewise cause reversible inflammation of vagus nerve causing such clinical presentations11). The history of relapsing symptom, followed by tapering of steroid, suggests additional evidence that inflammatory reaction contributed in the development of such symptom. Antiviral agent, such as acyclovir, is reported to be an effective treatment that we have used for facial palsy of the patient.
Another possibility can be suggested. Some reported rare anatomical variants of lower cranial nerves with interconnection between the glossopharyngeal nerve and vagus nerve. Such interconnection is so thin and very fragile that it makes them prone to shearing stress14). Though they have not suggested its clinical implication, it is possible that such interconnection holds some significance in the nerve conduction. As such, it may have been overlooked and damaged during the retraction of the surgery, causing lower cranial nerve palsy.
The clinical course of unilateral lower cranial nerve palsy is variable depending on the cause. Initial extensive laboratory work up with CT and MR imaging is necessary to evaluate the cause. If no particular structural abnormalities are noted, conservative treatment, including acyclovir and steroid administration with speech therapy, seems to be reasonable. If symptoms persist, surgical treatments, such as medialization laryngoplasty and laryngeal reinnervation, can be considered9,13).
We have experienced a very rare case of unilateral soft palate palsy, following MVD. Although the exact cause of this complication had not been identified, we have suggested possible causes for such rare complication. It is important to keep in mind that MVD is performed to improve the quality of life rather than to save life. Although it has relatively little complications, rare and debilitating complications must not be overlooked, and caution must be taken to avoid them.
MVD is a very effective and relatively safe surgical modality in the treatment of HFS. However, rare debilitating complications are reported, such as cranial nerve dysfunctions. The lower cranial nerve palsy we are reporting is one of such complication that can markedly decrease the quality of life of a patient and it is important to note that such delayed complication can occur without evident injury to the nerve during the operation. It is important to consider various etiologies as the treatment, and prognosis differs greatly on the cause. Additionally, careful surgical technique with minimal retraction with extra care to avoid damage to the lower cranial nerve during exploration may be important to minimize such complication.
References
1. Cohen-Gadol AA. Microvascular decompression surgery for trigeminal neuralgia and hemifacial spasm: naunces of the technique based on experiences with 100 patients and review of the literature. Clin Neurol Neurosurg. 2011; 113:844–853. PMID: 21752534.

2. Erman AB, Kejner AE, Hogikyan ND, Feldman EL. Disorders of cranial nerves IX and X. Semin Neurol. 2009; 29:85–92. PMID: 19214937.

3. Fang TJ, Tam YY, Courey MS, Li HY, Chiang HC. Unilateral high vagal paralysis: relationship of the severity of swallowing disturbance and types of injuries. Laryngoscope. 2011; 121:245–249. PMID: 21271569.

4. Gillig PM, Sanders RD. Cranial Nerves IX, X, XI, and XII. Psychiatry (Edgmont). 2010; 7:37–41. PMID: 20532157.
5. Jeon CJ, Kong DS, Lee JA, Park K. The efficacy and safety of microvascular decompression for hemifacial spasm in elderly patients. J Korean Neurosurg Soc. 2010; 47:442–445. PMID: 20617090.

6. Lapresle J, Faux N. [Unilateral involvement of IX, X, Xi and XII in cervical zoster. Cranial nerve contribution to vascular pathology]. J Neurol Sci. 1981; 52:351–357. PMID: 7310438.
7. Lapresle J, Lasjaunias P, Thévenier D. [Transitory paralysis of cranial nerves IX, X and XII as well as the left VII after angiography. Contribution to the ischemic pathology of the cranial nerves]. Rev Neurol (Paris). 1980; 136:787–791. PMID: 7209243.
8. Nusbaum AO, Som PM, Dubois P, Silvers AR. Isolated vagal nerve palsy associated with a dissection of the extracranial internal carotid artery. AJNR Am J Neuroradiol. 1998; 19:1845–1847. PMID: 9874534.
9. Paniello RC, Edgar JD, Kallogjeri D, Piccirillo JF. Medialization versus reinnervation for unilateral vocal fold paralysis: a multicenter randomized clinical trial. Laryngoscope. 2011; 121:2172–2179. PMID: 21898419.

10. Resnick DK, Jannetta PJ. Hyperactive rhizopathy of the vagus nerve and microvascular decompression. Case report. J Neurosurg. 1999; 90:580–582. PMID: 10067935.

11. Rhee DJ, Kong DS, Park K, Lee JA. Frequency and prognosis of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 2006; 148:839–843. discussion 843. PMID: 16804640.

12. Tang SC, Jeng JS, Liu HM, Yip PK. Isolated vagus nerve palsy probably associated with herpes simplex virus infection. Acta Neurol Scand. 2001; 104:174–177. PMID: 11551239.

13. Thakar A, Sikka K, Verma R, Preetam C. Cricothyroid approximation for voice and swallowing rehabilitation of high vagal paralysis secondary to skull base neoplasms. Eur Arch Otorhinolaryngol. 2011; 268:1611–1616. PMID: 21739100.

14. Tubbs RS, Mortazavi MM, Loukas M, Shoja MM, Cohen-Gadol AA. Intraoperative and anatomical descriptions of intracranial connections between the glossopharyngeal and vagus nerves: clinical implications. J Neurosurg. 2011; 115:179–181. PMID: 21395388.

Fig. 1
Preoperative MRI showing compression of the left facial nerve by anterior inferior cerebellar artery at the nerve root entry zone (offending artery is indicated by the arrow). No other structural abnormalities are noted. A: 3D T2 VISTA image. B: 3D TOF MR angiography image.
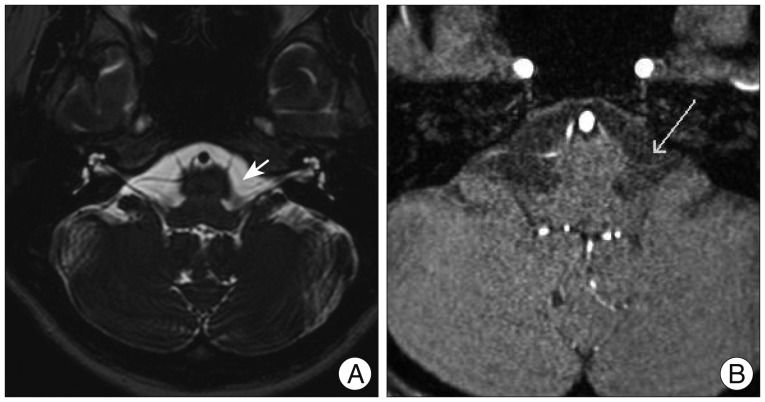
Fig. 2
Endoscopic evaluation of the larynx and vocal cord. A: Symmetric and mobile vocal cord. B: Uvula deviation to the right showing functional impairment of tensor and/or levator veli palatini muscle which is innervated by the vagus nerve.
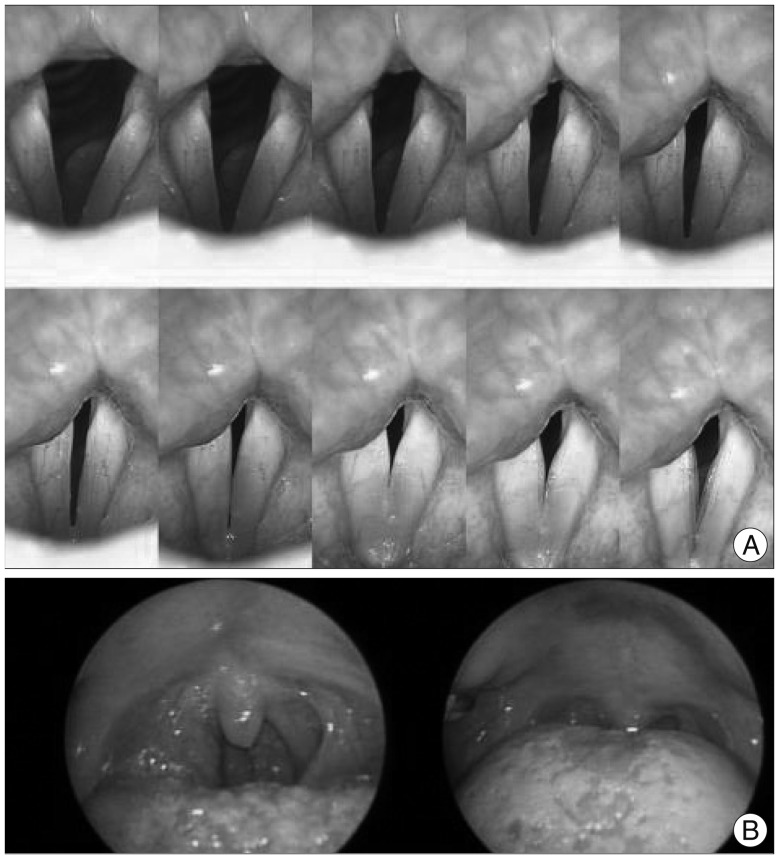
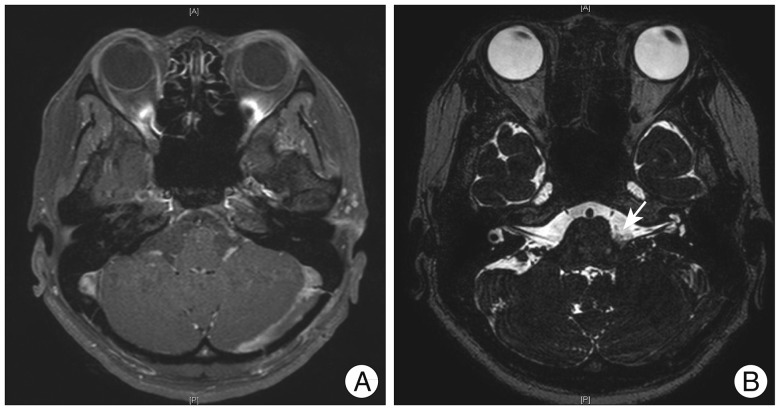
Fig. 3
A: Postoperative cranial nerve MRI showing encephalomalacia in the left cerebellar hemisphere, which is a postoperative change without evidence of significant abnormality along the pathway of intracranial portion of the lower cranial nerves. B: Postoperative T2 weighted image showing successful decompression with teflon felt placed between the offending artery and facial nerve (arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download